38482-71-2
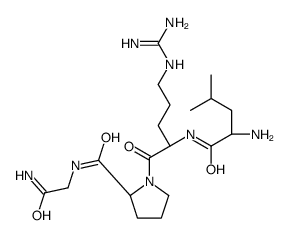
| Name | (2S)-1-[(2S)-2-[[(2S)-2-amino-4-methylpentanoyl]amino]-5-(diaminomethylideneamino)pentanoyl]-N-(2-amino-2-oxoethyl)pyrrolidine-2-carboxamide |
|---|---|
| Synonyms |
Glycinamide,L-leucyl-L-arginyl-L-prolyl
Lapg-NH2 Leucyl-arginyl-prolyl-glycinamide |
| Description | LH-RH (7-10) is a tetrapeptide, one of major degradation products of luteinising-hormone releasing hormone (LHRH) via pituitary and hypothalamus. LH-RH (7-10) produced in macrophages, type I-like and type II pneumocytes[3]. |
|---|---|
| Related Catalog | |
| In Vitro | The formation of LHRH 4-10, LHRH 7-10, and LHRH 6-10Co-incubation are inhibited by known enzyme inhibitors including Captopril (HY-B0368) (an ACE inhibitor), Thiorphan (HY-W013375) (an EP24.11 inhibitor), and Ethylenediaminetetraacetic acid (HY-Y0682) (an EP24.15 inhibitor)[3]. LH-RH (7-10) inhibits the binding of 125I-labeled GnRH to monoclonal antibody P81662 with an IC50 value of 1.7 nM[4]. |
| In Vivo | LH-RH (7-10) (i.v.) increases esterase activity in plasma kinin system in rats[1]. LH-RH (7-10) (ntracarotid infusion i.v.; single dose) stimulates a significant increase in plasma LH concentration in the jugular vein[2]. Animal Model: New Zealand White Rabbit[2] Dosage: 100, 500 and 2500 ng dissolved in 0-9% (w/v) NaCl solution and diluted Administration: Ntracarotid infusion; infused with a constant volume of 7-5 mL per 30 min Result: Caused LH release in the female rabbit during various reproductive conditions. |
| Molecular Formula | C19H36N8O4 |
|---|---|
| Molecular Weight | 440.54000 |
| Exact Mass | 440.28600 |
| PSA | 217.49000 |
| LogP | 2.35980 |