90779-69-4
| Name | Atosiban |
|---|---|
| Synonyms |
1-Deamino-2-D-Tyr-(O-ethyl)-4-Thr-8-ornoxytocin
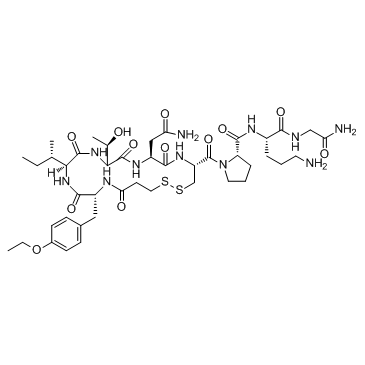
MFCD00672436 antocin ATOSIBAN ACETATE oxytocin,1-(3-mercaptopropanoicacid)-2-(o-ethyl-d-tyrosine)-4-l-threonine-8-l 1-(3-Mercaptopropanoic acid)-2-(O-ethyl-D-tyrosine)-4-L-threonine-8-L-ornithineoxytocin 1-deamino-2d-tyr-(oet)-4-thr-8-orn-oxytocin Tractocile 1-({(4R,7S,10S,13S,16R)-7-(2-Amino-2-oxoethyl)-13-[(2S)-butan-2-yl]-16-(4-ethoxybenzyl)-10-[(1R)-1-hydroxyethyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosan-4-yl}carbonyl)-L-prolyl-L-ornithylglycinamide 1-(3-Mercaptopropionic acid)-2-(3-(p-ethoxyphenyl)-D-alanine)-4-L-threonine-8-L-ornithineoxytocin Atosiban [Mpr-D-Tyr(OEt)-Ile-Thr-Asn-Cys]-Pro-Orn-Gly-NH2 1-({(4R,7S,10S,13S,16R)-7-(2-Amino-2-oxoethyl)-13-[(2S)-2-butanyl]-16-(4-ethoxybenzyl)-10-[(1R)-1-hydroxyethyl]-6,9,12,15,18-pentaoxo-1,2-dithia-5,8,11,14,17-pentaazacycloicosan-4-yl}carbonyl)-L-prolyl-L-ornithylglycinamide |
| Description | Atosiban(RW22164; Tractocile) is a nonapeptide, desamino-oxytocin analogue, and a competitive vasopressin/oxytocin receptor antagonist (VOTra). Atosiban inhibits the oxytocin-mediated release of inositol trisphosphate from the myometrial cell membrane.IC50 value:Target: As a result, there is reduced release of intracellular, stored calcium from the sarcoplasmic reticulum of myometrial cells, and reduced influx of Ca2+ from the extracellular space through voltage gated channels. In addition, atosiban suppresses oxytocin-mediated release of PGE and PGF from the decidua.[1][2] In human pre-term labour, atosiban, at the recommended dosage, antagonises uterine contractions and induces uterine quiescence. The onset of uterus relaxation following atosiban is rapid, uterine contractions being significantly reduced within 10 minutes to achieve stable uterine quiescence. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 1469.0±65.0 °C at 760 mmHg |
| Molecular Formula | C43H67N11O12S2 |
| Molecular Weight | 994.189 |
| Flash Point | 842.2±34.3 °C |
| Exact Mass | 993.441223 |
| PSA | 416.27000 |
| LogP | -3.41 |
| Vapour Pressure | 0.0±0.3 mmHg at 25°C |
| Index of Refraction | 1.549 |
| Storage condition | −20°C |
| Water Solubility | H2O: ≤100 mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | RS7590000 |