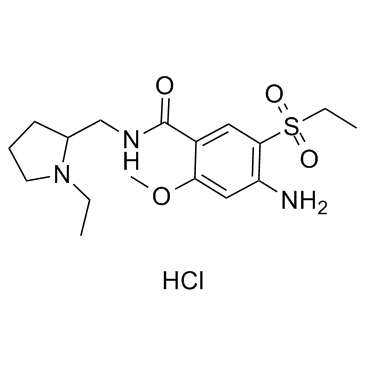
81342-13-4
| Name | Amisulpride hydrochloride |
|---|---|
| Synonyms |
Benzamide, 4-amino-N-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-2-methoxy-, hydrochloride (1:1)
4-Amino-N-[(1-ethyl-2-pyrrolidinyl)methyl]-5-(ethylsulfonyl)-2-methoxybenzamide hydrochloride (1:1) UNII:TC6N8QV7QO TC6N8QV7QO AMISULPRIDE HYDROCHLORIDE Amisulpride (hydrochloride) |
| Description | Amisulpride hydrochloride is a dopamine D2/D3 receptor antagonist with Kis of 2.8 and 3.2 nM for human dopamine D2 and D3, respectively. |
|---|---|
| Related Catalog | |
| Target |
Ki: 2.8 nM (D2 receptor), 3.2 nM (D3 receptor) |
| In Vitro | Amisulpride hydrochloride is an atypical dopamine D2/D3 receptor antagonist with Kis of 2.8 and 3.2 nM for human dopamine D2 and D3, respectively. Amisulpride hydrochloride (100 nM) inhibits quinpirole-elicited [3H]thymidine incorporation with an IC50 value of 22±3 nM (n=3). Amisulpride hydrochloride slightly but significantly increases [3H]dopamine release from slices of the rat striatum (S2/S1=0.88±0.04 under control conditions, n=6; 1.04±0.08 in the presence of 100 nM Amisulpride hydrochloride,n=4; P<0.05) and opposes the inhibitory effects of 7-OH-DPAT in both brain areas[1]. |
| In Vivo | Only the highest dose of Amisulpride hydrochloride (100 mg/kg) significantly reduces dopamine levels in the striatum or limbic system. Amisulpride hydrochloride significantly increases the synthesis of dopamine in the rat striatum and limbic system at doses of 20 and 100 mg/kg. Amisulpride hydrochloride (0.5 to 75 mg/kg) fails to provoke an additional increase in dopa accumulation in the striatum but slightly accelerates, at 75 mg/kg, dopamine synthesis in the limbic system. In comparison with vehicle-treated controls, Amisulpride hydrochloride (10 mg/kg) increases extracellular dopamine levels. The administration of Amisulpride hydrochloride (0.5 to 15 mg/kg s.c.) provokes a time- and dose-dependent increase in the stimulation-evoked dopamine release. Amisulpride hydrochloride decreases striatal ACh levels significantly at 30 and 100 mg/kg (87.5% and 56.3% of control levels, respectively)[1]. In both acute study, Amisulpride hydrochloride (70 mg/kg, p.o.) significantly increases the duration of swimming behavior [F(3,28)=45.90, p<0.01][2]. |
| Cell Assay | The functional effects of Amisulpride hydrochloride at the dopamine D3 receptor subtype are assessed. Briefly, the mitogenic response elicited in NG108-15 neuroblastoma-glioma cells stably transfected with human dopamine D3 receptor cDNA by the addition of 10 nM quinpirole in the presence of 1 μM forskolin is quantified by the incorporation of [3H]thymidine. Antagonism of quinpirole-induced mitogenesis is measured in the presence of increasing (0.1 to 100 nM) concentrations of Amisulpride hydrochloride[1]. |
| Animal Admin | A total of 64 male Swiss albino mice weighing between 20 to 30 g are used. The animals are fed with standard pellet diet and water ad libitum. The mice are divided in different groups (n=8 in each group) and drug administration is done as follows: Group 1 (control): distilled water (1 mL/kg) 23.5, 5 and 1 h before the test. Group 3 (Amisulpride hydrochloride): Amisulpride hydrochloride (70 mg/kg) 23.5, 5 and 1 h before the test[2]. |
| References |
| Molecular Formula | C17H28ClN3O4S |
|---|---|
| Molecular Weight | 405.940 |
| Exact Mass | 405.148895 |
| Storage condition | 2-8℃ |