1620458-09-4
| Name | Zoliflodacin |
|---|---|
| Synonyms |
FWL2263R77
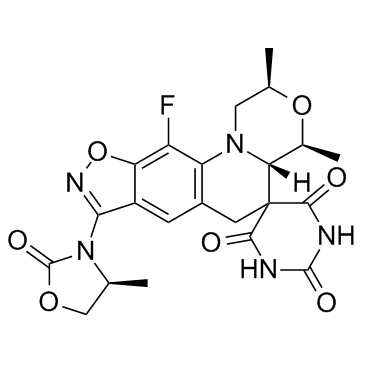
Zoliflodacin (2R,4S,4aS)-11-Fluoro-2,4-dimethyl-8-[(4S)-4-methyl-2-oxo-1,3-oxazolidin-3-yl]-1,2,4,4a-tetrahydro-2'H,6H-spiro[1,4-oxazino[4,3-a][1,2]oxazolo[4,5-g]quinoline-5,5'-pyrimidine]-2',4',6'(1'H,3'H)-trione |
| Description | Zoliflodacin (ETX0914;AZD0914) is a novel spiropyrimidinetrione bacterial DNA gyrase/topoisomerase inhibitor. Zoliflodacin has potent in vitro antibacterial activity against Gram-positive and Gram-negative organisms, including S. aureus with the MIC90 of 0.25 μg/mL. |
|---|---|
| Related Catalog | |
| Target |
MIC90: 0.25 μg/mL (S. aureus)[1] |
| In Vitro | Zoliflodacin has antibacterial activity against key Gram-positive (Staphylococcus aureus, Staphylococcus epidermidis, Streptococcus pneumoniae, Streptococcus pyogenes, and Streptococcus agalactiae), fastidious Gram-negative (Haemophilus influenzae, Neisseria gonorrhoeae), atypical (Legionella pneumophila), and anaerobic (Clostridium difficile) bacterial species, including isolates with known resistance to fluoroquinolones. The antibacterial activity of Zoliflodacin is shown to be via inhibition of DNA biosynthesis and accumulation of double-strand cleavages; this mechanism of action differs from those of other marketed antibacterial compounds, including fluoroquinolones. Zoliflodacin stabilizes and arrests the cleaved covalent complex of gyrase with double-strand broken DNA under permissive conditions and thus blocks religation of the double-strand cleaved DNA to form fused circular DNA[1]. |
| Cell Assay | Zoliflodacin broth macrodilution MICs are determined and used as the starting point for both in vitro time-kill and postantibiotic-effect (PAE) tests. In vitro static time-kill studies are conducted with glass tubes (18 by 150 mm, without agitation) containing 10-mL volumes of cation-adjusted Mueller-Hinton broth with logarithmically growing cultures (starting inoculum of 1×106 CFU/mL) against levofloxacin-susceptible and levofloxacin-resistant S. aureus. Zoliflodacin is tested at concentrations equivalent to 0.5, 1, 2, 4, and 8 times the MIC; samples are plated for colony counts at 0, 2, 4, 6, 8, and 24 h by using 100 μL aliquots spotted onto 25-ml sheep blood agar plates as described previously. Compounds are considered bactericidal at the lowest drug concentration that reduced viable organism counts by ≥3 log10 in 24 h. Time-kill studies are conducted in duplicate; tests are combined, and mean values are reported[1]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Molecular Formula | C22H22FN5O7 |
| Molecular Weight | 487.438 |
| Exact Mass | 487.150330 |
| LogP | 1.77 |
| Index of Refraction | 1.685 |
| Storage condition | 2-8℃ |