374559-48-5
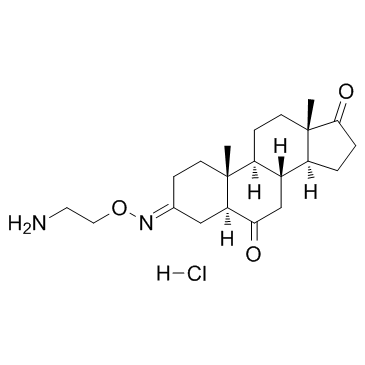
| Name | (5S,8R,9S,10R,13S,14S)-3-(2-aminoethoxyimino)-10,13-dimethyl-1,2,4,5,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthrene-6,17-dione,hydrochloride |
|---|---|
| Synonyms |
Androstane-3,6,17-trione,3-(O-(2-aminoethyl)oxime),hydrochloride (1:1)
Androstane-3,6,17-trione,3-(O-(2-aminoethyl)oxime),monohydrochloride,(5alpha) PST-2744 hydrochloride Istaroxime hydrochloride Istaroxime (hydrochloride) |
| Description | Istaroxime hydrochloride is a potent inhibitor of Na+,K+-ATPase with IC50 of 0.11 μM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 0.11 μM (Na+,K+-ATPase)[1] |
| In Vitro | Istaroxime hydrochloride acting as a positive inotropic compound through the inhibition of the Na+,K+-ATPase[2]. Istaroxime (PST2744) inhibits the Na+/K+-ATPase activity from dog kidney with an IC50 value of 0.43 ± 0.15 μM. Inhibition of Na+/K+-ATPase activity in preparations from guinea pig kidney yielded potencies of 8.5 μM for PST2744[3]. |
| In Vivo | Istaroxime (PST2744) induces a progressive increase in +dP/dtmax throughout the infusion that reaches 80% (ED80) at the cumulative dose of 1.89±0.37 mg/kg and a peak of 140±3.5% at the dose (EDmax) of 4.88±0.6 mg/kg[3]. |
| Kinase Assay | Dog or guinea pig kidney outer medulla is homogenized with a Polytron in 250 mM sucrose and 30 mM histidine, at pH 7.2. The homogenate is centrifuged at 6,000g for 15 min at 4°C and the supernatant at 48,000g for 30 min at 20°C with SDS and then layered onto a discontinuous sucrose density gradient (10, 15, and 29%) and centrifuged at 60,000 rpm for 115 min at 4°C. The pellet is resuspended in 25 mM imidazole and 1 mM EDTA, pH 7.5. Protein content is measured. Na+/K+-ATPase activity is measured after the release of 32P from [32P]ATP. Increasing concentrations of compounds are preincubated with purified enzyme for 10 min at 37°C in 120 μL of final volume of medium containing 140 mM NaCl, 3 mM MgCl2, 50 mM HEPES-Tris, and 3 mM ATP, pH 7.5. After preincubation, 10 μL of incubation solution containing 10 mM KCl and 20 nCi of [32P]ATP (0.5-3 Ci/mmol) is added, and the reaction is carried out for 15 min at 37°C before being stopped by acidification with 30% (v/v) perchloric acid. 32P is separated by centrifugation with activated charcoal and radioactivity measured by liquid scintillation counting. Inhibitory activity is expressed as percentage of control sample, carried out in the absence of standard compound. IC50 is calculated by weighed nonlinear regression curve fitting to the mass-action equilibrium[3]. |
| Animal Admin | Pigs[3] Male guinea Pigs (350-450 g) are used. Istaroxime (300 μg/kg) or Digoxin (75 μg/kg) are given by i.v. bolus 10 and 20 min before starting the exercise, respectively, and compared with vehicle. The following variables, HR, ECG, LVP, and aortic pressures, are recorded through a computerized acquisition system, which calculated the left ventricular rates of pressure changes. Data are analyzed from the real-time digitized recordings. Control values are obtained before compound administration. |
| References |
| Molecular Formula | C21H33ClN2O3 |
|---|---|
| Molecular Weight | 396.95100 |
| Exact Mass | 396.21800 |
| PSA | 81.75000 |
| LogP | 4.61080 |
| Storage condition | 2-8℃ |