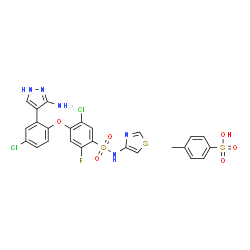
1430806-04-4
| Name | PF-05089771 |
|---|---|
| Synonyms |
4-[2-(3-Amino-1H-pyrazol-4-yl)-4-chlorophenoxy]-5-chloro-2-fluoro-N-4-thiazolyl-benzenesulfonamide 4-methylbenzenesulfonate (1:1)
PF-05089771 4-[2-(5-Amino-1H-pyrazol-4-yl)-4-chloro-phenoxy]-5-chloro-2-fluoro-N-thiazol-4-yl-benzenesulfonamide p-toluene sulfonate salt PF-05089771 p-toluene sulfonate salt MFCD30146405 4-[2-(3-Amino-1H-pyrazol-4-yl)-4-chlorophenoxy]-5-chloro-2-fluoro-N-(1,3-thiazol-4-yl)benzenesulfonamide 4-methylbenzenesulfonate (1:1) Benzenesulfonamide, 4-[2-(3-amino-1H-pyrazol-4-yl)-4-chlorophenoxy]-5-chloro-2-fluoro-N-4-thiazolyl-, 4-methylbenzenesulfonate (1:1) Pf-05089771 Tosylate |
| Description | PF-05089771 is a potent, state-dependent, subtype selective Nav1.7 inhibitor with IC50 of 11 nM, >1,000-fold selectivity over Nav1.3, 1.4 and Nav1.5, 1.8; interacts with the voltage-sensor domain (VSD) of domain IV, blocks all Nav1.7 splice variants with similar potency (5N11L, 5A11S, 5A11L and 5N11S, IC50s=11=33 nM); displays high selectivity over L-type calcium channels, KvLQT and hERG potassium channels; demonstrates in vivo efficacy in a mouse capsaicin-induced neurogenic flare model. Pain Phase 1 Discontinued |
|---|---|
| References | References 1. Swain NA, et al. J Med Chem. 2017 Aug 24;60(16):7029-7042. 2. Theile JW, et al. Mol Pharmacol. 2016 Nov;90(5):540-548. 3. Alexandrou AJ, et al. PLoS One. 2016 Apr 6;11(4):e0152405. 4. Chernov-Rogan T, et al. Proc Natl Acad Sci U S A. 2018 Jan 23;115(4):E792-E801. View Related Products by Target Sodium Channel Pain |
| Molecular Formula | C18H12Cl2FN5O3S2 |
|---|---|
| Molecular Weight | 672.556 |
| Exact Mass | 670.993652 |
| RIDADR | NONH for all modes of transport |
|---|