1018830-99-3
| Name | VCP171 |
|---|---|
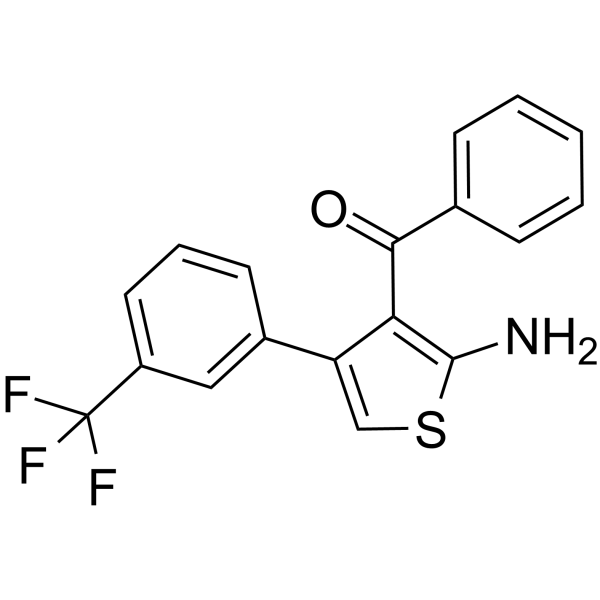
| Synonyms |
{2-Amino-4-[3-(trifluoromethyl)phenyl]-3-thienyl}(phenyl)methanone
VPC171 {2-amino-4-[3-(trifluoromethyl)phenyl]thiophen-3-yl}(phenyl)methanone VCP171 |
| Description | VCP171 is a potent adenosine A1 receptor (A1R) positive allosteric modulator (PAM). VCP171 is effective at decreasing excitatory synaptic currents in Lamina II of neuropathic pain model. VCP171 can be used for researching neuropathic pain[1]. |
|---|---|
| Related Catalog | |
| Target |
A1R |
| In Vivo | VCP171 (10 μM) reduces AMPAR-mediated evoked excitatory postsynaptic current (eEPSCs) in lamina I cells in sham and nerve-injured animals; increases paired pulse ration in cells from sham control animals; is significantly more effective in Lamina II neurons from nerve-injured animals than sham controls[1]. Animal Model: Neurons from male Sprague-Dawley rats (5-6 weeks; performed a partial nerve ligation (PNL) of the left sciatic nerve to create neuropathic pain model)[1] Dosage: 10 μM Administration: 0-30 min Result: Reduced AMPAR-mediated evoked excitatory postsynaptic current (eEPSCs) in Lamina I cells in sham (13±2%, n=7 cells) and nerve-injured animals (24±4%, n=8 cells) compared with predrug controls; increased paired pulse ration in cells from sham control animals; was significantly more effective in Lamina II neurons from nerve-injured animals than sham controls. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 507.0±50.0 °C at 760 mmHg |
| Molecular Formula | C18H12F3NOS |
| Molecular Weight | 347.354 |
| Flash Point | 260.4±30.1 °C |
| Exact Mass | 347.059174 |
| LogP | 5.30 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.599 |