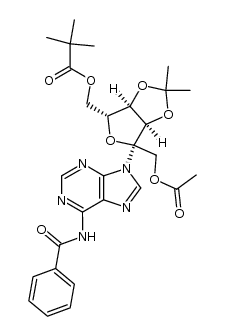
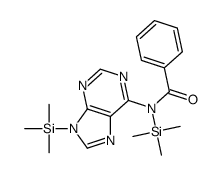
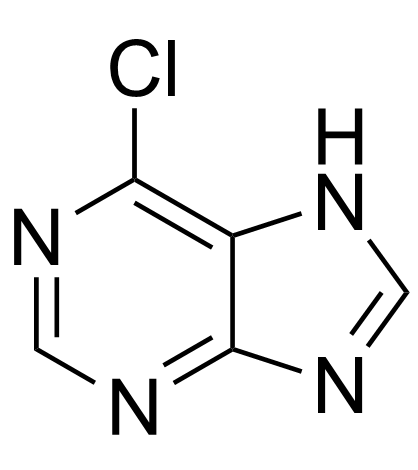
1874-54-0
| Name | (2R,3R,4S,5R)-2-(6-aminopurin-9-yl)-2,5-bis(hydroxymethyl)oxolane-3,4-diol |
|---|---|
| Synonyms |
Adenine,9beta-D-psicofuranosyl
6-Amino-9-D-psicofuranosylpurine Psicofuranosyladenine Angustmycin C psicofuranine angustmicine C |
| Description | Psicofuramine a nucleoside antibiotic and has the inhibition of xanthosine 5'-phosphate aminase. Psicofuranine also specifically inhibits GMP synthase, and interrupts parasite growth. Psicofuranine exhibits a dose-dependent inhibition of P. falciparum growth[1][2]. |
|---|---|
| Related Catalog | |
| Target |
Xanthosine 5'-phosphate aminase[2] P. falciparum[1] E. coli[1] |
| In Vitro | Psicofuranine specifically inhibits bacterial GMP synthase as demonstrated by isolation of Psicofuranine-resistant Escherichia coli mutants with mutations in the gene encoding GMP synthase and inhibition of bacterial growth. Psicofuranine exhibits a dose-dependent inhibition of P. falciparum growth with an IC50 of 0.3 mM. The Psicofuranine inhibitory concentration is similar to that of E. coli[1]. |
| References |
| Density | 2.02g/cm3 |
|---|---|
| Boiling Point | 720.1ºC at 760 mmHg |
| Molecular Formula | C11H15N5O5 |
| Molecular Weight | 297.26700 |
| Flash Point | 389.3ºC |
| Exact Mass | 297.10700 |
| PSA | 159.77000 |
| Vapour Pressure | 9.24E-22mmHg at 25°C |
| Index of Refraction | 1.855 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~90% 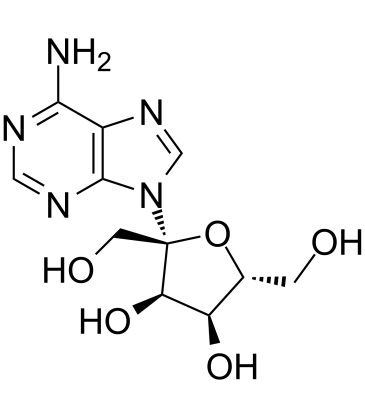
1874-54-0 |
| Literature: Mahmood; Vasella; Bernet Helvetica Chimica Acta, 1991 , vol. 74, # 7 p. 1555 - 1584 |
|
~50% 
1874-54-0 |
| Literature: Grouiller; Chattopadhyaya 1984 , vol. 38, # 5 B p. 367 - 373 |
|
~74% 
1874-54-0 |
| Literature: Mahmood; Vasella; Bernet Helvetica Chimica Acta, 1991 , vol. 74, # 7 p. 1555 - 1584 |
|
~% 
1874-54-0 |
| Literature: Mahmood; Vasella; Bernet Helvetica Chimica Acta, 1991 , vol. 74, # 7 p. 1555 - 1584 |
|
~% 
1874-54-0 |
| Literature: Mahmood; Vasella; Bernet Helvetica Chimica Acta, 1991 , vol. 74, # 7 p. 1555 - 1584 |
|
~% 
1874-54-0 |
| Literature: Grouiller; Chattopadhyaya 1984 , vol. 38, # 5 B p. 367 - 373 |
|
~% 
1874-54-0 |
| Literature: Grouiller; Chattopadhyaya 1984 , vol. 38, # 5 B p. 367 - 373 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |