1616428-23-9
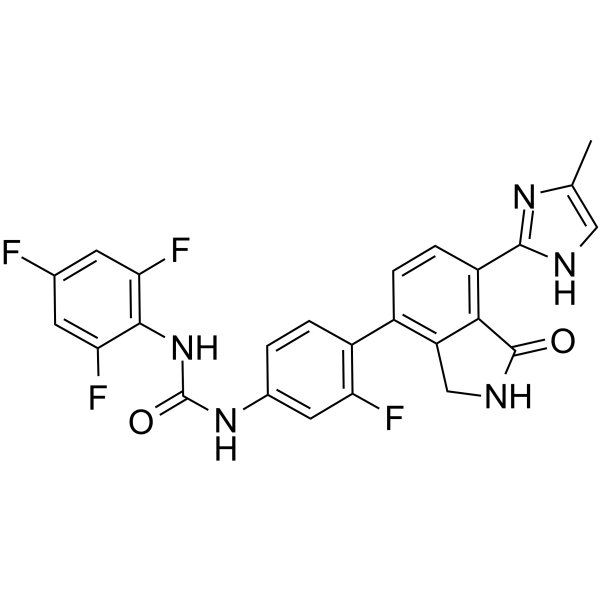
| Name | 1-{3-Fluoro-4-[7-(4-methyl-1H-imidazol-2-yl)-1-oxo-2,3-dihydro-1H-isoindol-4-yl]phenyl}-3-(2,4,6-trifluorophenyl)urea |
|---|---|
| Synonyms | 1-{3-Fluoro-4-[7-(4-methyl-1H-imidazol-2-yl)-1-oxo-2,3-dihydro-1H-isoindol-4-yl]phenyl}-3-(2,4,6-trifluorophenyl)urea |
| Description | Luxeptinib (CG-806) is an orally active, reversible, first-in-class, non-covalent and potent pan-FLT3/pan-BTK inhibitor. Luxeptinib induces cell cycle arrest, apoptosis or autophagy in acute myeloid leukemia cells[1][2][3][4]. |
|---|---|
| Related Catalog | |
| Target |
Pan-FLT3/Pan-BTK[1] |
| In Vitro | Luxeptinib (MEC-1 CLL cells; 0.1~10 µM; 72 hours) inhibits cells proliferation with an IC50 of 32 nM[1]. Luxeptinib inhibits BCR signaling-induced phosphorylation of BTK, PLCg2, AKT, ERK1/2, S6 ribosomal protein and strongly suppresses SYK phosphorylation in primary chronic lymphocytic leukemia (CLL) cells[1]. Luxeptinib (MV4-11 cells; 500 pM; 1 hour) completely inhibits phosphorylation of FLT3 and STAT5[2]. Cell Proliferation Assay[1] Cell Line: MEC-1 CLL cells Concentration: 0.1~10 µM Incubation Time: 72 hours Result: Inhibited cells proliferation with an IC50 of 32 nM. |
| References |
[4]. Aptose Biosciences to Present CG’806 Data at AACR Hematologic Malignancies Meeting |
| Density | 1.5±0.1 g/cm3 |
|---|---|
| Boiling Point | 663.8±55.0 °C at 760 mmHg |
| Molecular Formula | C25H17F4N5O2 |
| Molecular Weight | 495.43 |
| Flash Point | 355.3±31.5 °C |
| Exact Mass | 495.131836 |
| LogP | 4.44 |
| Vapour Pressure | 0.0±2.0 mmHg at 25°C |
| Index of Refraction | 1.674 |
| Storage condition | 2-8°C |