1350624-75-7
| Name | Margetuximab |
|---|
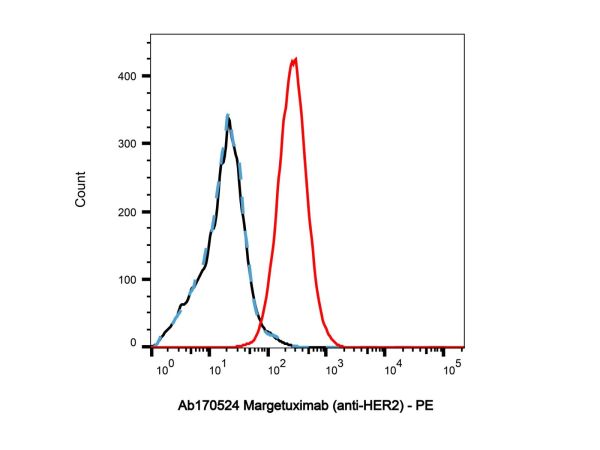
| Description | Margetuximab (MGAH22) is a chimeric anti-HER2 monoclonal antibody optimized Fc domain, with an EC50 value of 39.33 ng/mL. Margetuximab can be used for researching metastatic HER2-positive breast cancer[1]. |
|---|---|
| Related Catalog | |
| Target |
EC50: 39.33 ng/mL (HER2)[1] |
| In Vitro | Margetuximab (MGAH22) enhances the antibody-dependent cell-mediated cytotoxicity activity of effector cells expressing the CD16A-158F variant[1]. Cell Proliferation Assay Cell Line: JIMT-1, MCF-7, ZR-75-1, SKBR-3, HT-29, SW750 and N87[1] Concentration: 0.001-1000 ng/mL Incubation Time: 6 days Result: Enhances the antibody-dependent cell-mediated cytotoxicity activity of effector cells expressing the CD16A-158F variant. |
| In Vivo | Margetuximab (2-4 mg/kg; IP 5 or 6 times at weekly) can firstly and significantly reduces the tumor size at day 30 - 37 in mice model[1]. Margetuximab (15-150 mg/kg; IV; 6 weekly) exhibits well tolerated in cynomolgus monkeys, decreases NK cells by an average of 51%, and induces IL-6 release[1]. Margetuximab (50 mg/kg; IV; single dosage) exhibits favorable safety profile[1]. Pharmacokinetic Parameters of Margetuximab in cynomolgus monkeys[1]. Male, IV (50 mg/kg) Female, IV (50 mg/kg) Cmax (mg/mL) 1.62 ± 0.10 1.70 ± 0.14 AUC0-¥ (mg·hour/mL) 294.1 ± 53.2 314.2 ± 31.3 T1/2β (days) 9.3 ± 1.8 9.7 ± 1.1 Clearance (mL/hour) 0.43 ± 0.07 0.40 ± 0.04 VSS (mL) 132 ± 2 127 ± 8 |
| References |
| No Any Chemical & Physical Properties |