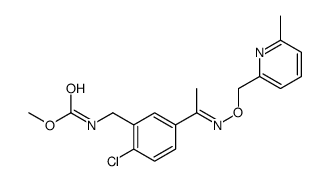
799247-52-2
| Name | pyribencarb |
|---|---|
| Synonyms |
Pyribencarb
methyl {2-chloro-5-[(1E)-1-(6-methyl-2-pyridylmethoxyimino)ethyl]benzyl}carbamate methyl [(2-chloro-5-{(1E)-N-[(6-methylpyridin-2-yl)methoxy]ethanimidoyl}phenyl)methyl]carbamate methyl N-[[2-chloro-5-[(1E)-1-[[(6-methyl-2-pyridinyl)methoxy]imino]ethyl]phenyl]methyl]carbamate methyl N-[[2-chloro-5-[(Z)-C-methyl-N-[(6-methylpyridin-2-yl)methoxy]carbonimidoyl]phenyl]methyl]carbamate |
| Description | Pyribencarb is a benzylcarbamate-type fungicide, which is active against a wide range of plant pathogenic fungi. Pyribencarb is a potent Qo inhibitor of cytochrome b. Pyribencarb is especially active against Botrytis cinerea and Sclerotinia sclerotirum[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Pyribencarb potently inhibits succinate-cytochrome c reductase (SCR) activities of Botrytis cinerea (cucumber gray mold), Corynespora cassiicola (leaf spot) and decylubiquinol-cytochrome c reductase (UCR) activity of B. cinerea. Pyribencarb inhibits the UCR of B. cinerea in an uncompetitive manner, and the substrate-dependent inhibition constant is found from calculation to be 13 nM[1]. The target site of Pyribencarb is cytochrome b of complex III in the electron transport system of the respiratory chain. The inhibitory potency of pyribencarb on SCR activities of plants, rats and carp is relatively weak compared with that of strobilurin fungicides[1]. |
| References |
| Density | 1.221g/cm3 |
|---|---|
| Molecular Formula | C18H20ClN3O3 |
| Molecular Weight | 361.82300 |
| Exact Mass | 361.11900 |
| PSA | 76.30000 |
| LogP | 4.04460 |
| Index of Refraction | 1.57 |
| Storage condition | 2-8°C |
| RIDADR | NONH for all modes of transport |
|---|