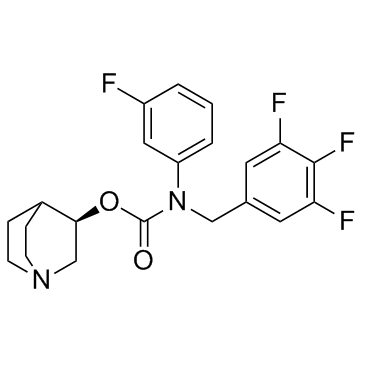
385367-47-5
| Name | [(3R)-1-azabicyclo[2.2.2]octan-3-yl] N-(3-fluorophenyl)-N-[(3,4,5-trifluorophenyl)methyl]carbamate |
|---|---|
| Synonyms |
Tarafenacin
UNII-LDV98UN52Y Carbamic acid, N-(3-fluorophenyl)-N-[(3,4,5-trifluorophenyl)methyl]-, (3R)-1-azabicyclo[2.2.2]oct-3-yl ester (3R)-1-Azabicyclo[2.2.2]oct-3-yl (3-fluorophenyl)(3,4,5-trifluorobenzyl)carbamate (3R)-1-Az2,4(1Habicyclo(2.2.2)octan-3-yl (3-fluorophenyl)((3,4,5-trifluorophenyl)methyl)carbamate Tarafenacin [INN] |
| Description | Tarafenacin(SVT-40776) is a highly selective M3 muscarinic receptor antagonist (Ki= 0.19 nM), ~200 fold selectivity over M2 receptor.IC50 value: 0.19 nM (Ki) [1]Target: M3 muscarinic receptorin vitro: SVT-40776 is highly selective for M(3) over M(2) receptors (Ki = 0.19 nmol.L(-1) for M(3) receptor affinity). SVT-40776 was the most potent in inhibiting carbachol-induced bladder contractions of the anti-cholinergic agents tested, without affecting atrial contractions over the same range of concentrations. SVT-40776 exhibited the highest urinary versus cardiac selectivity (199-fold) [1]. SVT-40776 has a much higher binding affinity (K(d) = 0.4 nM) to M5 mAChR than that of solifenacin (K(d) = 31 nM) with the same reeptor. The calculated binding free energy change (-2.3 ± 0.3 kcal/mol) from solifenacin to SVT-40776 is in good agreement with the experimentally derived binding free energy change (-2.58 kcal/mol), suggesting that our modeled M5 mAChR structure and its complexes with the antagonists are reliable [2].in vivo: In the guinea pig in vivo model, SVT-40776 inhibited 25% of spontaneous bladder contractions at a very low dose (6.97 microg.kg(-1) i.v), without affecting arterial blood pressure [1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 483.4±45.0 °C at 760 mmHg |
| Molecular Formula | C21H20F4N2O2 |
| Molecular Weight | 408.389 |
| Flash Point | 246.2±28.7 °C |
| Exact Mass | 408.146088 |
| PSA | 32.78000 |
| LogP | 4.59 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.587 |
| Storage condition | 2-8℃ |