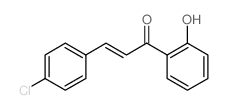
19275-70-8
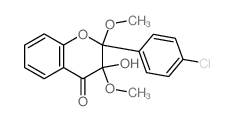
| Name | 2-(4-chlorophenyl)-3-hydroxychromen-4-one |
|---|---|
| Synonyms |
4'-chloro-3-hydroxyflavone
3-hydroxy-2-(4-chlorophenyl)-chromen-4(1H)-one 4'-Chloroflavonol 3-hydroxy-2-(4-chlorophenyl)-4H-chromen-4(1H)-one 3-Hydroxy-4'-chloroflavone 2-(4-chlorophenyl)-3-hydroxy-4H-chromen-4-one 4'-chloro-3-flavonol |
| Description | Tyrosinase-IN-4 (compound 34) is a potent inhibitor of tyrosinase. Tyrosinase is a copper-containing metalloenzyme that is responsible for the rate-limiting catalytic step in the melanin biosynthesis and enzymatic browning. Tyrosinase-IN-4 has the potential for the research of skin whitening agents and food preservatives[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.464g/cm3 |
|---|---|
| Boiling Point | 416.8ºC at 760 mmHg |
| Molecular Formula | C15H9ClO3 |
| Molecular Weight | 272.68 |
| Flash Point | 205.9ºC |
| Exact Mass | 272.02400 |
| PSA | 50.44000 |
| LogP | 3.81900 |
| Vapour Pressure | 1.08E-07mmHg at 25°C |
| Index of Refraction | 1.683 |
|
~99% 
19275-70-8 |
| Literature: Dhoubhadel, S. P.; Tuladhar, Sudersan M.; Tuladhar, Sarbajna M.; Wagley, Pradyumna P. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1981 , vol. 20, # 6 p. 511 - 512 |
|
~74% 
19275-70-8 |
| Literature: Rao, Sudhakar T.; Singh, Anil Kumar; Trivedi, Girish Kumar Heterocycles, 1984 , vol. 22, # 6 p. 1377 - 1382 |
|
~% 
19275-70-8 |
| Literature: Kurzwernhart, Andrea; Kandioller, Wolfgang; Bartel, Caroline; Baechler, Simone; Trondl, Robert; Muehlgassner, Gerhard; Jakupec, Michael A.; Arion, Vladimir B.; Marko, Doris; Keppler, Bernhard K.; Hartinger, Christian G. Chemical Communications, 2012 , vol. 48, # 40 p. 4839 - 4841 |
|
~% 
19275-70-8 |
| Literature: Prakash, Om; Pahuja, Saroj; Tanwar, Madan P Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1994 , vol. 33, p. 272 - 273 |
|
~% 
19275-70-8 |
| Literature: Zanarotti, Antonio Heterocycles, 1982 , vol. 19, # 9 p. 1585 - 1586 |
|
~% 
19275-70-8 |
| Literature: Dhoubhadel, S. P.; Tuladhar, Sudersan M.; Tuladhar, Sarbajna M.; Wagley, Pradyumna P. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1981 , vol. 20, # 6 p. 511 - 512 |
|
~% 
19275-70-8 |
| Literature: Dhoubhadel, S. P.; Tuladhar, Sudersan M.; Tuladhar, Sarbajna M.; Wagley, Pradyumna P. Indian Journal of Chemistry, Section B: Organic Chemistry Including Medicinal Chemistry, 1981 , vol. 20, # 6 p. 511 - 512 |
|
~% 
19275-70-8 |
| Literature: Rao, Takkellapati Sudhakar; Trivedi, Girish Kumar Heterocycles, 1987 , vol. 26, # 8 p. 2117 - 2124 |
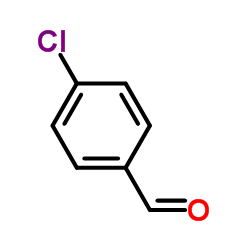
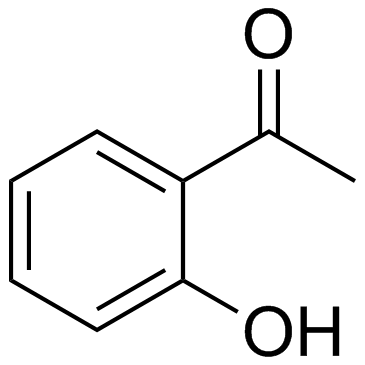
| Precursor 6 | |
|---|---|
| DownStream 1 | |