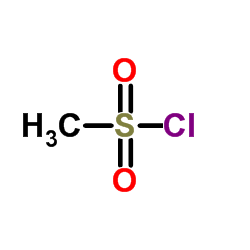
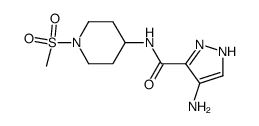
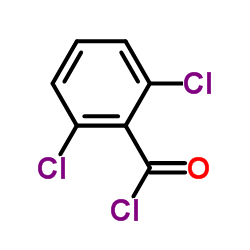
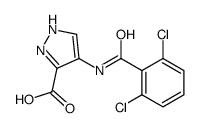
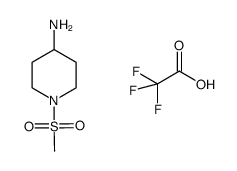
902156-99-4
| Name | 4-[(2,6-dichlorobenzoyl)amino]-N-(1-methylsulfonylpiperidin-4-yl)-1H-pyrazole-5-carboxamide |
|---|---|
| Synonyms |
CS-0840
AT 9311 4-(2,6-dichlorobenzoylamino)-1H-pyrazole-3-carboxylic acid (1-methanesulphonyl-piperidin-4-yl)amide NVP LCQ195 LCQ 195 NVP-LCQ195 |
| Description | NVP-LCQ195 (AT9311; LCQ195) is a small molecule heterocyclic inhibitor of CDK1, CDK2, CDK3 and CDK5 with IC50 of 1-42 nM.IC50 Value: 1 nM(CDK5/p25 and CDK5/p35); 2 nM(CDK1/cyclinB and CDK2/cyclinA); 5 nM(CDK2/cyclinE); 42 nM(CDK3/cyclinE)Target: CDKsLCQ195 induced cell cycle arrest and eventual apoptotic cell death of MM cells, even at sub-lmol/l concentrations, spared non-malignant cells, and overcame the protection conferred to MM cells by stroma or cytokines of the bone marrow milieu. In MM cells, LCQ195 triggered decreased amplitude of transcriptional signatures associated with oncogenesis, drug resistance and stem cell renewal, including signatures of activation of key transcription factors for MM cells e.g. myc, HIF-1a, IRF4. Bortezomib-treated MM patients whose tumours had high baseline expression of genes suppressed byLCQ195 had significantly shorter progression-free and overall survival than those with low levels of these transcripts in their MM cells. These observations provide insight into the biological relevance of multi-targeted CDK inhibition in MM. |
|---|---|
| Related Catalog | |
| Target |
Cdk5/p25:1 nM (IC50) CDK5/p35:1 nM (IC50) Cdk1/cyclin B:2 nM (IC50) cdk2/cyclin A:2 nM (IC50) CDK2/cyclinE:5 nM (IC50) CDK9/cyclinT1:15 nM (IC50) CDK3/Cyclin E:42 nM (IC50) cdk6/cyclin D3:187 nM (IC50) CDK7/Cyclin H/MAT1:3564 nM (IC50) |
| References |
| Molecular Formula | C17H19Cl2N5O4S |
|---|---|
| Molecular Weight | 460.33500 |
| Exact Mass | 459.05300 |
| PSA | 136.13000 |
| LogP | 3.91620 |
| Storage condition | -20°C |
|
~93% 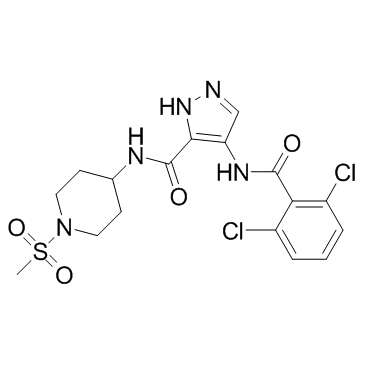
902156-99-4 |
| Literature: ASTEX THERAPEUTICS LIMITED Patent: WO2008/1101 A2, 2008 ; Location in patent: Page/Page column 366-367 ; |
|
~% 
902156-99-4 |
| Literature: ASTEX THERAPEUTICS LIMITED Patent: WO2008/1101 A2, 2008 ; Location in patent: Page/Page column 369-370 ; |
|
~49% 
902156-99-4 |
| Literature: ASTEX THERAPEUTICS LIMITED Patent: WO2007/129066 A1, 2007 ; Location in patent: Page/Page column 97-98 ; |