25161-41-5
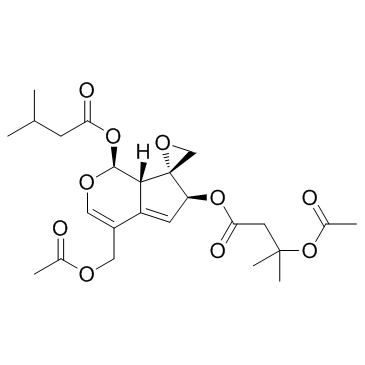
| Name | [(1S,6S,7R,7aS)-4-(acetyloxymethyl)-1-(3-methylbutanoyloxy)spiro[6,7a-dihydro-1H-cyclopenta[c]pyran-7,2'-oxirane]-6-yl] 3-acetyloxy-3-methylbutanoate |
|---|---|
| Synonyms |
Acevaltrate
(1S,6S,7R,7aS)-4-(Acetoxymethyl)-1-[(3-methylbutanoyl)oxy]-6,7a-dihydro-1H-spiro[cyclopenta[c]pyran-7,2'-oxiran]-6-yl 3-acetoxy-3-methylbutanoate Acetoxyvaltrate EINECS 246-685-1 1,7a-Dihydro-1,6-dihydroxyspiro(cyclopenta[c]pyran-7(6H),2'-oxirane)-4-methanol 4-Acetate 1(or 6)-Isovalerate 6(or 1)-(3-Hydroxy-3-methylbutyrate Acetate) Butanoic acid, 3-(acetyloxy)-3-methyl-, (1S,6S,7R,7aS)-4-[(acetyloxy)methyl]-6,7a-dihydro-1-(3-methyl-1-oxobutoxy)spiro[cyclopenta[c]pyran-7(1H),2'-oxiran]-6-yl ester Acevaltratum 3-(Acetyloxy)-3-methylbutanoic acid (1S-(1a,6a,7b,7aa))-4-((Acetyloxy)methyl)-6,7a-dihydro-1-(3-methyl-1-oxobutoxy)spiro(cyclopenta[c]pyran-7(1H),2'-oxiran)-6-yl Ester Acetovaltrate (1S,6S,7R,7aS)-4-[(acetyloxy)methyl]-1-[(3-methylbutanoyl)oxy]-6,7a-dihydro-1H-spiro[cyclopenta[c]pyran-7,2'-oxiran]-6-yl 3-(acetyloxy)-3-methylbutanoate |
| Description | Acevaltrate, isolated from Valeriana glechomifolia, inhibits the Na+/K+-ATPase activity in the rat kidney and brain hemispheres with IC50s of 22.8±1.1 μM and 42.3±1.0 μM, respectively[1]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 22.8±1.1 μM (Na+/K+-ATPase, in rat kidney), 42.3±1.0 μM (Na+/K+-ATPase, in rat brain hemispheres)[1] |
| In Vitro | Acevaltrate differentiallys inhibit the activity of rat P-type ATPases in vitro. 60.7±7.3% inhibition of the rat H+/K+-ATPase is achieved at 100 µM[1]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 538.7±50.0 °C at 760 mmHg |
| Molecular Formula | C24H32O10 |
| Molecular Weight | 480.505 |
| Flash Point | 229.0±30.2 °C |
| Exact Mass | 480.199554 |
| PSA | 126.96000 |
| LogP | 1.91 |
| Vapour Pressure | 0.0±1.4 mmHg at 25°C |
| Index of Refraction | 1.531 |
| Storage condition | 2-8C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|