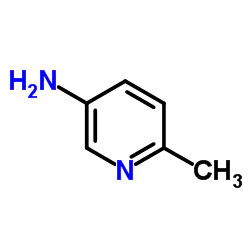
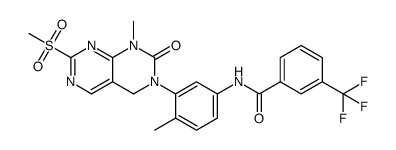
839706-07-9
| Name | N-[4-methyl-3-[1-methyl-7-[(6-methylpyridin-3-yl)amino]-2-oxo-4H-pyrimido[4,5-d]pyrimidin-3-yl]phenyl]-3-(trifluoromethyl)benzamide |
|---|---|
| Synonyms |
N-(4-Methyl-3-{1-methyl-7-[(6-methyl-3-pyridinyl)amino]-2-oxo-1,4-dihydropyrimido[4,5-d]pyrimidin-3(2H)-yl}phenyl)-3-(trifluoromethyl)benzamide
2H-1,4-Benzothiazine,urea deriv. GNF-7 |
| Description | GNF-7 inhibits Bcr-Abl WT and Bcr-Abl T315I with IC50 of 133 nM and 61 nM, respectively. IC50 value: 133 nM (Bcr-Abl WT), 61 nM (Bcr-Abl T315I)Target: Bcr-Ablin vitro: GNF-7 is amongst the first type II inhibitors capable of inhibiting T315I to be described and will serve as a valuable lead to design next generation Bcr-Abl kinase inhibitors. GNF-7 exhibits some selectivity (4 to 100-fold) for T315I Bcr-Abl (IC50 = 11 nM, in Ba/F3 cell line) relative to kinases such as TPR-Met, NPM-ALK, JAK-3, Flt-3. in vivo: GNF-7 displays significant efficacy against T315I-Bcr-Abl without appreciable toxicity in a bioluminescent xenograft mouse model using a transformed T315I-Bcr-Abl-Ba/F3 cell line that has a stable luciferase expression. GNF-7 exhibits excellent pharmacokinetic parameters in mice, with good systemic exposure (AUC = 26656 hrs*nM, Cmax = 3.6 uM) along with reasonable half life (t1/2=3.2 hrs) and favorable oral bioavailability (BAV=36%) being observed following oral administration of a single dose of 20 mg/kg. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Molecular Formula | C28H24F3N7O2 |
| Molecular Weight | 547.531 |
| Exact Mass | 547.194336 |
| PSA | 106.84000 |
| LogP | 2.78 |
| Appearance | white solid |
| Index of Refraction | 1.655 |
| Storage condition | -20℃ |
| Precursor 2 | |
|---|---|
| DownStream 0 | |