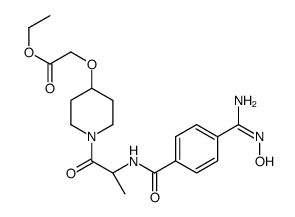
172927-65-0
| Name | ethyl 2-[1-[(2S)-2-[[4-[(Z)-N'-hydroxycarbamimidoyl]benzoyl]amino]propanoyl]piperidin-4-yl]oxyacetate |
|---|---|
| Synonyms |
Xubix
UNII-YUE443B0NF Sibrafiban Ro 48-3657 |
| Description | Sibrafiban (RO 48-3657) is the orally active, nonpeptide, double-prodrug of Ro 44-3888 and a selective glycoprotein IIb/IIIa receptor antagonist. Sibrafiban inhibits platelet aggregation[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Glycoprotein IIb/IIIa receptor[1] |
| In Vitro | The effects of site occupancy by Sibrafiban on platelet activation are assessed using P-selectin expression, fibrinogen binding and microaggregate formation. Sibrafiban inhibits ADP and TRAP-stimulated fibrinogen binding and microaggregate formation in a concentration-dependent manner, whereas P-selectin expression is relatively unaltered. A decrease in site occupancy from peak to trough of Sibrafiban does not result in increased activation of platelets[3]. |
| In Vivo | The effects of Ro 44-3888 on the platelet aggregation response to ADP (17 μmol) and on cutaneous bleeding times is determined in 8 rhesus monkeys given Sibrafiban 0.25 mg/kg/day or 0.5 mg/kg/day orally for 8 days. The maximum inhibition of ex vivo platelet aggregation and prolongation of bleeding time by Ro 44-3888 are dose dependent[1]. |
| References |
[1]. M Dooley, et al. Sibrafiban. Drugs. 1999 Feb;57(2):225-30; discussion 231-2. |
| Density | 1.33g/cm3 |
|---|---|
| Molecular Formula | C20H28N4O6 |
| Molecular Weight | 420.46000 |
| Exact Mass | 420.20100 |
| PSA | 143.55000 |
| LogP | 1.49920 |
| Index of Refraction | 1.598 |