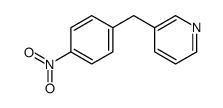
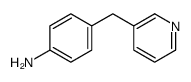
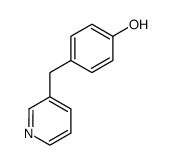
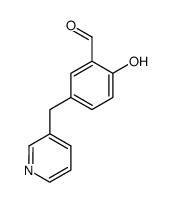
85666-17-7
| Name | furegrelate sodium |
|---|---|
| Synonyms |
Sodium 5-(pyridin-3-ylmethyl)-1-benzofuran-2-carboxylate
Sodium p-Styrenesulfonate 4-Vinylbenzenesulfonic acid sodium salt sodium styrene sulphonate p-styrenesulfonic acid sodium salt sodium styrene sulfonate sodium styrene-4-sulphonate Sodium 5-(3-pyridinylmethyl)-1-benzofuran-2-carboxylate Styrene-4-sulfonic acid sodium salt 5-(3-Pyridinylmethyl)-2-benzofurancarboxylic Acid Sodium Salt 2-Benzofurancarboxylic acid, 5-(3-pyridinylmethyl)-, sodium salt (1:1) sodium 4-styrenesulfonate Sodium 4-vinylbenzenesulfonate |
| Description | Furegrelate Sodium (U-63557A) is a potent, orally available, and selective thromboxane synthase inhibitor. Furegrelate Sodium inhibits human platelet microsomal thromboxane A2 (TxA2) synthase with an IC50 of 15 nM. Furegrelate Sodium is being developed as an antiplatelet agent[1][2]. |
|---|---|
| Related Catalog | |
| In Vivo | Furegrelate Sodium (U-63557A) (1-5 mg/kg; Oral) prevents blockage of the coronary artery[1]. Furegrelate Sodium (0.1-5 mg/kg; i.v.) prevents the blockage of stenosed coronary arteries caused platelet aggregation[1]. Furegrelate Sodium blunts the development of hypoxia-induced pulmonary arterial hypertension (PAH) in an established neonatal piglet model primarily by preserving the structural integrity of the pulmonary vasculature[2]. Furegrelate also is orally available, has a long half-life of 4.2–5.8 hours in adult humans (compared to several other therapies for PAH including nitric oxide and prostacyclin analogs), and reportedly is highly specific for its target enzyme[2]. Animal Model: Mongrel dogs (19-30 kg)[1] Dosage: 1-5 mg/kg Administration: Oral (via a gastric tube) Result: Prevented blockage of the coronary artery. |
| References |
| Boiling Point | 458.7ºC at 760 mmHg |
|---|---|
| Molecular Formula | C15H10NNaO3 |
| Molecular Weight | 275.235 |
| Flash Point | 231.2ºC |
| Exact Mass | 275.055847 |
| PSA | 66.16000 |
| LogP | 1.78210 |
|
~96% 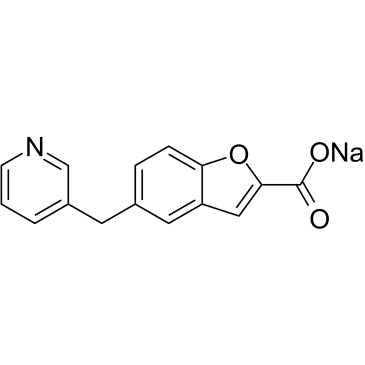
85666-17-7 |
| Literature: Johnson; Nidy; Aiken; Crittenden; Gorman Journal of medicinal chemistry, 1986 , vol. 29, # 8 p. 1461 - 1468 |
|
~% 
85666-17-7 |
| Literature: Johnson; Nidy; Aiken; Crittenden; Gorman Journal of medicinal chemistry, 1986 , vol. 29, # 8 p. 1461 - 1468 |
|
~% 
85666-17-7 |
| Literature: Johnson; Nidy; Aiken; Crittenden; Gorman Journal of medicinal chemistry, 1986 , vol. 29, # 8 p. 1461 - 1468 |
|
~% 
85666-17-7 |
| Literature: Johnson; Nidy; Aiken; Crittenden; Gorman Journal of medicinal chemistry, 1986 , vol. 29, # 8 p. 1461 - 1468 |
|
~% 
85666-17-7 |
| Literature: Johnson; Nidy; Aiken; Crittenden; Gorman Journal of medicinal chemistry, 1986 , vol. 29, # 8 p. 1461 - 1468 |
| Precursor 5 | |
|---|---|
| DownStream 0 | |