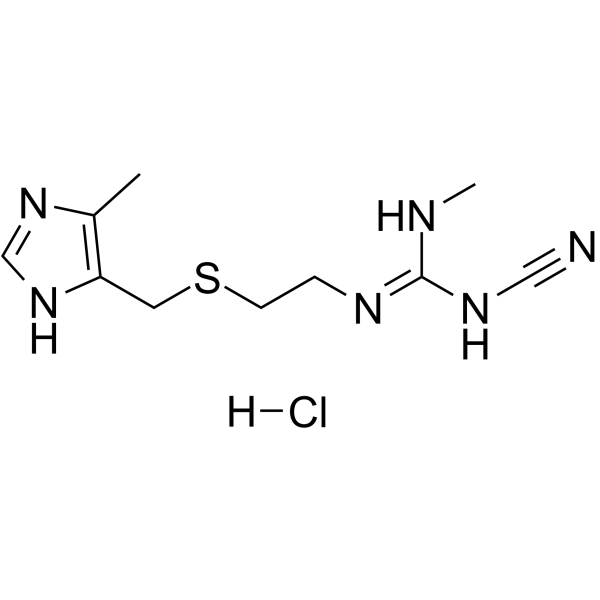
70059-30-2
| Name | cimetidine hydrochloride |
|---|---|
| Synonyms |
EINECS 274-297-2
MFCD01724314 Cimetidin-HCl Cimetidine Hydrochloride USP24 Cimetidinhydrochlorid cimetex Cimetidine hydrochloride |
| Description | Cimetidine (SKF-92334) hydrochloride is an orally active and inverse histamine H2 receptor antagonist with a Ki of 0.6 μM. Cimetidine hydrochloride is a gastric acid reducer, and can be used for duodenal and gastric ulcers research. Cimetidine hydrochloride has anti-cancer and anti-inflammatory activity[1][2][5]. |
|---|---|
| Related Catalog | |
| Target |
H2 Receptor:0.6 μM (Kd) |
| In Vitro | Cimetidine (SKF-92334) hydrochloride, a partial agonist for H2R, has a pharmacological profile different from ranitidine and famotidine, possibly contributing to its antitumor activity on gastrointestinal cancers [1]. Cimetidine hydrochloride has no effect on the uptake and cytotoxicity of cisplatin in ovarian cancer cells with high OCT2 mRNA levels (IGROV-1 cells)[3]. Cimetidine hydrochloride shows no effect on proliferation, survival, migration and invasion of 3LL cells. Cimetidine hydrochloride reverses MDSC-mediated T-cell suppression, and improves IFN-γ production[4]. Cimetidine-mediated down-regulation of NCAM involved suppression of the nuclear translocation of NF-kappaB, a transcriptional activator of NCAM gene expression[5]. |
| In Vivo | Cimetidine (SKF-92334) hydrochloride reduces CD11b(+)Gr-1(+) myeloid derived-suppressive cell (MDSC) accumulation in spleen, blood and tumor tissue of tumor-bearing mice[4]. Cimetidine hydrochloride exerts a beneficial effect on periodontal disease in rats, decreasing the RANKL/OPG ratio in gingival connective tissue and reducing alveolar bone resorption[6]. |
| References |
| Boiling Point | 488ºC at 760 mmHg |
|---|---|
| Molecular Formula | C10H17ClN6S |
| Molecular Weight | 288.80000 |
| Flash Point | 248.9ºC |
| Exact Mass | 288.09200 |
| PSA | 114.19000 |
| LogP | 2.18118 |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
MUTATION DATA
|
| RIDADR | NONH for all modes of transport |
|---|---|
| RTECS | MF0035100 |
| HS Code | 2933290090 |
| HS Code | 2933290090 |
|---|---|
| Summary | 2933290090. other compounds containing an unfused imidazole ring (whether or not hydrogenated) in the structure. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |