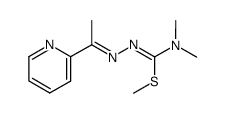
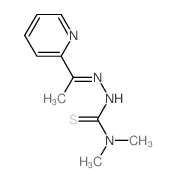
79514-49-1
| Name | 1-λ1-selanyl-N,N-dimethyl-N'-[(E)-1-pyridin-2-ylethylideneamino]methanimidamide |
|---|---|
| Synonyms |
2-acetylpyridine-N,N-dimethylselenosemicarbazone
2-acetylpyridine 4,4-dimethyl-3-selenosemicarbazone 2-Acetylpyridine 4,4-dimethylselenosemicarbazone |
| Description | Ap44mSe is a selenosemicarbazone that effectively depletes cellular Fe, resulting in transferrin receptor-1 up-regulation, ferritin down-regulation, and increased expression of the potent metastasis suppressor, N-myc downstream regulated gene-1. Ap44mSe forms redox active Cu complexes that target the lysosome to induce lysosomal membrane permeabilization[1]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C10H15N4Se |
|---|---|
| Molecular Weight | 270.21300 |
| Exact Mass | 271.04600 |
| PSA | 40.52000 |
| LogP | 0.71610 |
|
~% 
79514-49-1 |
| Literature: Klayman; Scovill; Bartosevich; Mason European Journal of Medicinal Chemistry, 1981 , vol. 16, # 4 p. 317 - 320 |
|
~% 
79514-49-1 |
| Literature: Klayman; Scovill; Bartosevich; Mason European Journal of Medicinal Chemistry, 1981 , vol. 16, # 4 p. 317 - 320 |
| Precursor 2 | |
|---|---|
| DownStream 0 | |