53066-26-5
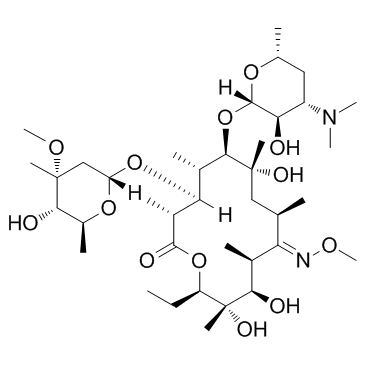
| Name | (E)-9-deoxo-9-methoxyiminoerythromycin A |
|---|---|
| Synonyms |
(E)-9-chloro-4-methyl-8-(tetrahydro-2H-pyran-2-yloxy)-4-nonenoic acid
4-Nonenoic acid,9-chloro-4-methyl-8-[(tetrahydro-2H-pyran-2-yl)oxy] |
| Description | Lexithromycin is an erythromycin A derivative, with antibacterial activity. |
|---|---|
| Related Catalog | |
| Target |
Bacterial[1] |
| In Vitro | Lexithromycin is an erythromycin A derivative, with antibacterial activity. Lexithromycin shows minimal inhibitory concentration (MIC) of 0.06 μg/mL against S. pyogenes CN10A and Streptococcus sp. 64/848C, 0.25 μg/mL against Staphylococcus aureus Oxford, 0.5 μg/mL against S. aureus Russell and S. aureus T2, 4 μg/mL against S. pyogenes CN10A and Haemophilus influenzae Wy 21[1]. |
| References |
[1]. Hunt E, et al. Erythromycin A 11,12-methylene acetal. J Antibiot (Tokyo). 1988 Nov;41(11):1644-8. |
| Density | 1.26g/cm3 |
|---|---|
| Boiling Point | 824.6ºC at 760mmHg |
| Molecular Formula | C38H70N2O13 |
| Molecular Weight | 762.96800 |
| Flash Point | 452.5ºC |
| Exact Mass | 762.48800 |
| PSA | 198.43000 |
| LogP | 2.21890 |
| Index of Refraction | 1.543 |
| Storage condition | 2-8℃ |
|
~% 
53066-26-5 |
| Literature: Everett, Jeremy R.; Hatton, Ian K.; Hunt, Eric; Tyler, John W.; Williams, David J. Journal of the Chemical Society, Perkin Transactions 2: Physical Organic Chemistry (1972-1999), 1989 , p. 1719 - 1728 |