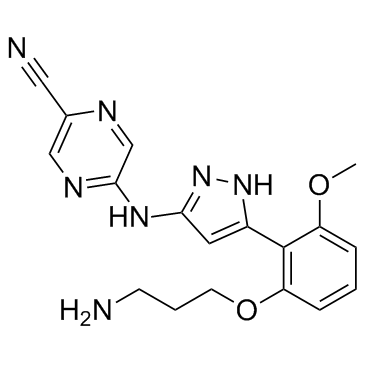
1234015-52-1
| Name | 5-(5-(2-(3-aminopropoxy)-6-methoxyphenyl)-1H-pyrazol-3-ylamino)pyrazine-2-carbonitrile |
|---|---|
| Synonyms |
prexasertib
ly 2606368 5-({5-[2-(3-Aminopropoxy)-6-methoxyphenyl]-1H-pyrazol-3-yl}amino)-2-pyrazinecarbonitrile LY2606368 |
| Description | Prexasertib (LY2606368) is a potent, selective and ATP-competitive checkpoint kinase 1 (Chk1) inhibitor, with an IC50 and a Ki of <1 nM and 0.9 nM, respectively. |
|---|---|
| Related Catalog | |
| Target |
Chk1:<1 nM (IC50) Chk2:8 nM (IC50) Chk1:0.9 nM (Ki) |
| In Vitro | Prexasertib (LY2606368) is a potent and selective ATP competitive inhibitor of Chk1, with an IC50 of <1 nM, and also inhibits CHK2, with an IC50 of 8 nM. Prexasertib has an EC50 of 1 nM for CHK1 activity through autophosphorylation of serine 296 and <31 nM for HT-29 CHK2 autophosphorylation (S516). Prexasertib potently abrogates the G2-M checkpoint activated by doxorubicin in p53-deficient HeLa cells with an EC50 of 9 nM. However, 100 nM Prexasertib does not inhibit PMA-stimulated RSK but instead weakly stimulates phosphorylation of S6 on serines 235/236. Prexasertib is broadly antiproliferative with IC50s of 3 nM, 3 nM, 10 nM, 37 nM, and 68 nM against U-2 OS, Calu-6, HT-29, HeLa, and NCI-H460 cell lines, respectively. Prexasertib (4 nM) results in a large shift in cell-cycle populations from G1 and G2-M to S-phase with an accompanied induction of H2AX phosphorylation in U-2 OS cells[1]. Prexasertib (LY2606368; 25 μM) exhibits inhibitory activities against proliferation of AGS and MKN1 cells. Prexasertib (20 nM) inhibits HR repair capacity DR-GFP cells. Prexasertib (5 nM) in combination with PARP inhibitor BMN673, displays synergistic anticancer effects in gastric cancer cells[2]. |
| In Vivo | Prexasertib (LY2606368; 15 mg/kg, s.c.) significantly inhibits tumor growth in xenograft tumor models with less animal weight loss[1]. Prexasertib (LY2606368; 2 mg/kg, s.c.) and BMN673 combination has synergistic anticancer effect in gastric cancer PDX model, and the effect is higher than that of one drug alone[2]. |
| Cell Assay | Proliferation inhibition effect of Chk1 ablation, IR sensitivity, anticancer effect of BMN673 and Prexasertib are detected by MTS Cell Proliferation Colorimetric Assay Kit. Cells are seeded into 96 wells cell culture plate, then treated with indicated experiment conditions, then added 20 μL MTS reagent to each well subsequently, after incubated for 2 hours, cell viability of each well is detected on microplate reader at a wavelength of 490 nM[2]. |
| Animal Admin | Female CD-1 nu-/nu- mice (26-28 g) are used for this study. Tumor growth is initiated by subcutaneous injection of 1×106 Calu-6 cells in a 1:1 mixture of serum-free growth medium and Matrigel in the rear flank of each subject animal. When tumor volumes reach approximately 150 mm3 in size, the animals are randomized by tumor size and body weight, and placed into their respective treatment groups. Vehicle consisting of 20% Captisol pH4 or Prexasertib is administered by subcutaneous injection in a volume of 200 μL. Four, eight, 12, 24, and 48 hours after drug administration, blood for plasma drug exposure is extracted via cardiac puncture and assayed on a Sciex API 4000 LC/MS-MS system. The xenograft tissue is promptly removed and prepared. Lysates are analyzed by immunoblot analysis for protein phosphorylation levels. Group means, SEs and P values are calculated using Kronos. |
| References |
| Density | 1.4±0.1 g/cm3 |
|---|---|
| Boiling Point | 608.5±55.0 °C at 760 mmHg |
| Molecular Formula | C18H19N7O2 |
| Molecular Weight | 365.389 |
| Flash Point | 321.8±31.5 °C |
| Exact Mass | 365.160034 |
| PSA | 134.76000 |
| LogP | 2.03 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.655 |