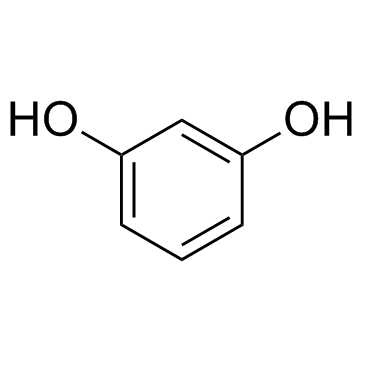
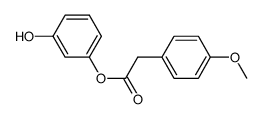
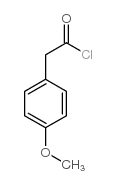
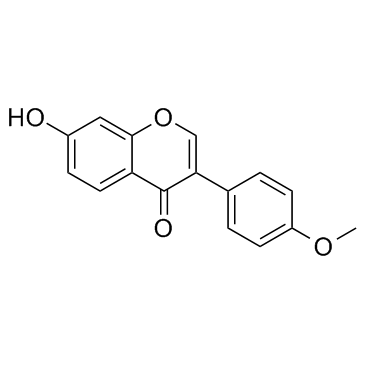
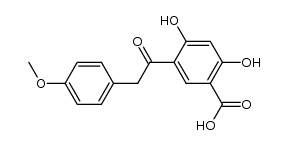
487-49-0
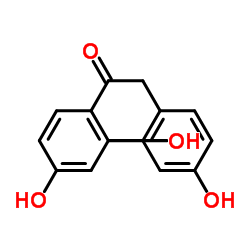
| Name | 1-(2,4-dihydroxyphenyl)-2-(4-methoxyphenyl)ethanone |
|---|---|
| Synonyms |
Phenobarbital sodium salt
2,4-dihydroxy-4'-methoxy deoxybenzoin |
| Description | Ononetin, a natural deoxybenzoin, is a potent and selective TRPM3 channel blocker with an IC50 of 0.3 μM[1]. |
|---|---|
| Related Catalog | |
| Target |
TRPM3:0.3 μM (IC50) |
| In Vitro | Ononetin (1-10 μM) completely and reversibly abrogates Ca2+ entry and ionic currents through recombinantly expressed TRPM3α2 and block the pregnenolone sulphate-inducible Ca2+ entry in primary cultures of mouse or rat dorsal root ganglia (DRG) neurones, indicating biological activity towards endogenously expressed TRPM3[1]. Oxidative intermediates of Ononetin may be involved in activating TRPA1, but not in the block of TRPM3 by Ononetin[1]. |
| In Vivo | Ononetin (10 mg/kg, i.p.) treatment completely reverses established Freund's Complete Adjuvant (FCA)-induced heat hypersensitivity, demonstrating that the loss of hypersensitivity in Trpm3-/- mice is unlikely to be caused by developmental or compensatory mechanisms and suggests that TRPM3 may be a tractable target for inflammatory pain[2]. |
| References |
| Density | 1.275g/cm3 |
|---|---|
| Boiling Point | 464.7ºC at 760mmHg |
| Melting Point | 158.0-162.0°C |
| Molecular Formula | C15H14O4 |
| Molecular Weight | 258.26900 |
| Flash Point | 176.9ºC |
| Exact Mass | 258.08900 |
| PSA | 66.76000 |
| LogP | 2.53180 |
| Vapour Pressure | 2.95E-09mmHg at 25°C |
| Index of Refraction | 1.62 |
| HS Code | 2914509090 |
|---|
|
~10% 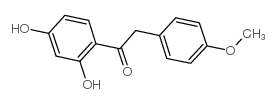
487-49-0 |
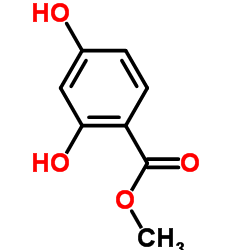
| Literature: Waehaelae, Kristiina; Hase, Tapio A. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1991 , # 12 p. 3005 - 3008 |
|
~55% 
487-49-0 |
| Literature: HANBUL COSMETIC CO., LTD. Patent: WO2005/54169 A1, 2005 ; Location in patent: Page/Page column 8; 9 ; |
|
~94% 
487-49-0 |
| Literature: Pazenok, Sergii; Lui, Norbert Patent: US2010/121082 A1, 2010 ; Location in patent: Page/Page column 2 ; |
|
~% 
487-49-0 |
| Literature: Walz Justus Liebigs Annalen der Chemie, 1931 , vol. 489, p. 118,127, 140 |
|
~% 
487-49-0 |
| Literature: Virtanen; Hietala Acta Chemica Scandinavica (1947-1973), 1958 , vol. 12, p. 579 Full Text Show Details Bradbury; White Journal of the Chemical Society, 1951 , p. 3447 Full Text Show Details Bose; Siddiqui Journal of Scientific and Industrial Research, 1951 , vol. 10 B, p. 291,292 |
|
~% 
487-49-0 |
| Literature: Whalley Journal of the Chemical Society, 1957 , p. 1833,1834 |
|
~% 
487-49-0 |
| Literature: Whalley Journal of the Chemical Society, 1957 , p. 1833,1834 |
|
~% 
487-49-0 |
| Literature: Baker; Eastwood Journal of the Chemical Society, 1929 , p. 2907 |
|
~% 
487-49-0 |
| Literature: Whalley Journal of the Chemical Society, 1957 , p. 1833,1834 |
| Precursor 9 | |
|---|---|
| DownStream 10 | |
| HS Code | 2914509090 |
|---|---|
| Summary | HS:2914509090 other ketones with other oxygen function VAT:17.0% Tax rebate rate:9.0% Supervision conditions:none MFN tariff:5.5% General tariff:30.0% |