88755-39-9
| Name | 2,4-bis(1,3-benzodioxol-5-yl)-4-oxobutanoic acid |
|---|---|
| Synonyms |
2,4-Bis-aziridin-1-yl-6-chlor-pyrimidin
Ethymidine Aethimidinum 2,4-Diethylenimino-6-chloropyrimidine 2,4-bis-aziridin-1-yl-6-chloro-pyrimidine Etimidin Ethimidine 2,6-Diethylenimino-4-chloropyrimidine Aethimidinium |
| Description | RUNX1/ETO tetramerization-IN-1 is a small-molecule inhibitor of RUNX1/ETO tetramerization, exhibits anti-leukemic effect. RUNX1/ETO tetramerization-IN-1 specifically targets to NHR2 of RUNX1/ETO (EC50=0.25 μM), restores gene expression down-regulated by RUNX1/ETO. RUNX1/ETO tetramerization-IN-1 inhibits the proliferation of RUNX1/ETO-depending SKNO-1 cells, and reduces the RUNX1/ETO-related tumor growth in a mouse model[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
IC50: 630 μM (RUNX1-NHR2 tetramerization)[1] |
| In Vitro | RUNX1/ETO is composed by the DNA-binding Runt-domain5, the product of the RUNX1 gene, and by four nervy homology regions (NHR1-4), the product of the ETO gene. The NHR2 domain is responsible for the tetramerization of RUNX1/ETO. RUNX1/ETO tetramerization-IN-1 (compound 7.44) (1 μM and 10 μM; 3, 5, 7 d) selectively reduces the viability of RUNX1/ETO-dependent human leukemic SKNO-1 cells instead of U937 cells[1]. RUNX1/ETO tetramerization-IN-1 (compound 7.44) (25 μM and 50 μM; 5 d) inhibits the growth of and induces myeloid differentiation in RUNX1/ETO-expressing cells (SKNO-1, Kasumi-1, and K562)[2]. RUNX1/ETO tetramerization-IN-1 (100 μM; 7 d) induces growth-arrest and differentiation of RUNX1/ETOtr-expressing CD34+ progenitor cells[2]. RUNX1/ETO tetramerization-IN-1 (compound 7.44) has favorable physicochemical and ADME properties with high aqueous solubility, high stability in buffer and plasma, and a low hepatic intrinsic clearance in vitro, with the aqueous solubility of 60 μg/mL[3]. RUNX1/ETO tetramerization-IN-1 (1 μM and 10 μM) shows a potential to inhibit CYP2B6, 2C9, 2C19, and 3A4[3]. RUNX1/ETO tetramerization-IN-1 (compound 8) (50 μM; 16 h) inhibits c-Jun N-terminal kinase (JNK) and affect the JNK-pathway in cells[4]. Cell Viability Assay[1] Cell Line: RUNX1/ETO-dependent human leukemic SKNO-1 and U937 cells Concentration: 1 μM and 10 μM Incubation Time: 3, 5, 7 days Result: Inhibited the SKNO-1 cell growth specifically. Cell Viability Assay[3] Cell Line: Pharmacokinetic properties of RUNX1/ETO tetramerization-IN-1 Concentration: Incubation Time: Result: Kinetic solubility (99% PBS, 1% DMSO) 177 µM Plasma protein binding (mouse plasma, 60 min) 98.4% Plasma stability (mouse plasma, 0–240 min) No degradation Hepatocyte stability (mouse hepatocytes) 2.5 µL/min/million cells Chemical stability in PBS (0–4 h) No degradation |
| In Vivo | RUNX1/ETO tetramerization-IN-1 (compound 7.44) (200-250 μg/kg; i.p.; 5 times per week; 130 d) delays tumor growth of RUNX1/ETO cells in mice[2]. Animal Model: NSG immunodeficient mice (NOD.Cg-Prkdcscid Il2rgtm1WjI/SzJ) injected with Kasumi-1 cells[2] Dosage: 200-250 μg/Kg Administration: Intraperitoneal injection; 5 times per week, for 130 days Result: Reduced the dissemination of leukemic cells, remained 75% mice alive at day 130 post-treatment. |
| References |
| Density | 1.472g/cm3 |
|---|---|
| Boiling Point | 598.6ºC at 760 mmHg |
| Molecular Formula | C18H14O7 |
| Molecular Weight | 342.30000 |
| Flash Point | 223.2ºC |
| Exact Mass | 342.07400 |
| PSA | 91.29000 |
| LogP | 2.58520 |
| Index of Refraction | 1.641 |
|
~% 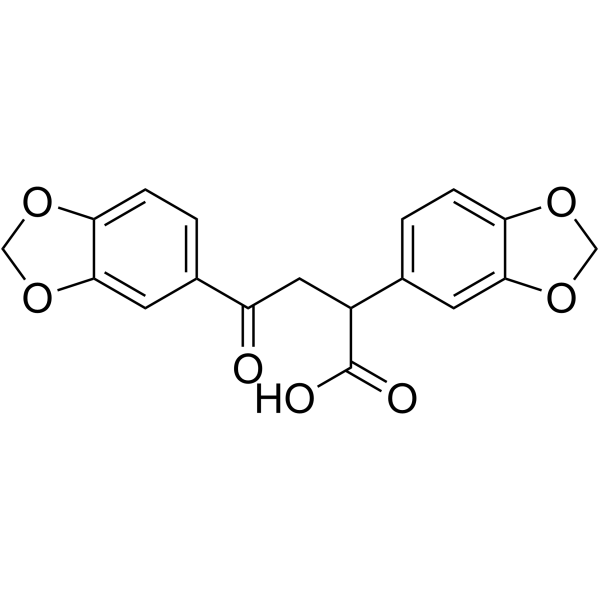
88755-39-9 |
| Literature: Ishii; Kawanabe; Harada; et al. Chemical and Pharmaceutical Bulletin, 1983 , vol. 31, # 9 p. 3039 - 3055 |
| Precursor 1 | |
|---|---|
| DownStream 1 | |
![6-(1,3-benzodioxol-5-yl)-7,8-dihydro-6H-benzo[f][1,3]benzodioxol-5-one structure](https://image.chemsrc.com/caspic/189/88775-69-3.png)