690270-29-2
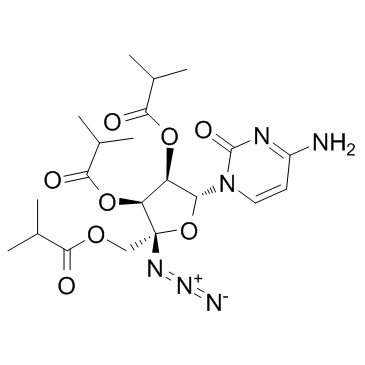
| Name | isobutyric acid (2R,3S,4R,5R)-5-(4-amino-2-oxo-2H-pyrimidin-1-yl)-2-azido-4-isobutyryloxy-2-isobutyryloxymethyltetrahydrofuran-3-yl ester |
|---|---|
| Synonyms | Balapiravir |
| Description | Balapiravir (R1626, Ro 4588161) is the prodrug of a nucleoside analogue inhibitor of the hepatitis C virus (HCV) RNA-dependent RNA polymerase (R1479, RG1479).IC50 Value: Target: HCVBalapiravir(R-1626; R 1626; Ro 4588161) is useful for Anti HCV. Balapiravir (R1626) is the tri-isobutyrate ester prodrug of R1479 under clinical development to improve exposure of R1479 upon oral administration. Balapiravir was discontinued for safety reasons in 28-36% of patients (most often for lymphopenia) and the percentage of patients with serious adverse events (especially hematological, infection, ocular events) was dose related. Serious hematological adverse events (particularly neutropenia, lymphopenia) were more common in balapiravir recipients. Two deaths in the balapiravir/peginterferon alfa-2a/ribavirin combination groups were considered possibly related to study medication. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C21H30N6O8 |
|---|---|
| Molecular Weight | 494.49800 |
| Exact Mass | 494.21300 |
| PSA | 198.79000 |
| LogP | 1.72966 |
| Storage condition | 2-8℃ |