52598-04-6
| Name | n-hexyl-1,1-d2 alcohol |
|---|
| Description | 1-Hexanol-d3 is the deuterium labeled 1-Hexanol[1]. 1-Hexanol, a primary alcohol, is a surfactant that can be employed in industrial processes to enhance interfacial properties[2]. 1-Hexanol uncouples mitochondrial respiration by a non-protonophoric mechanism[3]. |
|---|---|
| Related Catalog | |
| In Vitro | Stable heavy isotopes of hydrogen, carbon, and other elements have been incorporated into drug molecules, largely as tracers for quantitation during the drug development process. Deuteration has gained attention because of its potential to affect the pharmacokinetic and metabolic profiles of drugs[1]. |
| References |
| Molecular Formula | C6H12D2O |
|---|---|
| Molecular Weight | 104.18700 |
| Exact Mass | 104.11700 |
| PSA | 20.23000 |
| LogP | 1.55900 |
|
~98% 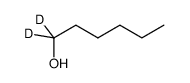
52598-04-6 |
| Literature: Duffault, Jean-Marc; Hanoteau, Pascal; Parrilla, Alfredo; Einhorn, Jacques Synthetic Communications, 1996 , vol. 26, # 17 p. 3257 - 3265 |
|
~91% 
52598-04-6 |
| Literature: Fujita, Kazuya; Yorimitsu, Hideki; Shinokubo, Hiroshi; Oshima, Koichiro Journal of the American Chemical Society, 2004 , vol. 126, # 21 p. 6776 - 6783 |
|
~95% 
52598-04-6 |
| Literature: Schwab, John M.; Habib, Asif; Klassen, John B. Journal of the American Chemical Society, 1986 , vol. 108, # 17 p. 5304 - 5308 |
|
~% 
52598-04-6 |
| Literature: Boden, Neville; Bushby, Richard J.; Clark, Leslie D. Journal of the Chemical Society, Perkin Transactions 1: Organic and Bio-Organic Chemistry (1972-1999), 1983 , p. 543 - 551 |
|
~% 
52598-04-6 |
| Literature: Makra, Ferenc; Rohloff, John C.; Muehldorf, A. V.; Link, John O. Tetrahedron Letters, 1995 , vol. 36, # 38 p. 6815 - 6818 |