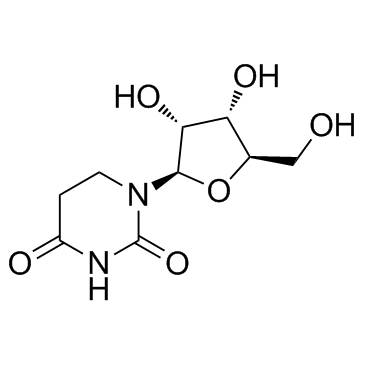
18771-50-1
| Name | tetrahydrouridine |
|---|---|
| Synonyms |
4-Hydroxy-1-(β-D-ribofuranosyl)tetrahydro-2(1H)-pyrimidinone
Tetrahydro-uridin UNII:0NIZ8H6OL8 3,4,5,6-tetrahydrouridine |
| Description | Tetrahydrouridine is potent inhibitor of cytidine deaminase (CDA), which competitively blocks the enzyme's active site more effectively than intrinsic cytidine. |
|---|---|
| Related Catalog | |
| Target |
cytidine deaminase (CDA)[1] |
| In Vitro | Tetrahydrouridine (THU) is a specific inhibitor of cytidine deaminase (CDA) which can suppress deamination in the catabolism of cytotoxic deoxycytidine analogues like ara-C and Gemcitabine. To test how Tetrahydrouridine affects the Gemcitabine-mediated anti-neoplastic effect on pancreatic and lung carcinoma cells, a combination therapy is performed. As expected, high CDA expression in BxPC-3 and H441 results in improved Gemcitabine sensitivity after a 100 µM Tetrahydrouridine treatment. The sensitivity of BxPC-3 and H441 cell lines increases by as much as approximately 2.1 and 4.4 fold respectively. On the other hand, MIAPaCa-2 and H1299 cells unexpectedly become more sensitive to Gemcitabine with low CDA expression. MIAPaCa-2 and H1299 cells show a change in IC50 of 2.2 and 2.3 fold respectively. However, Panc-1 and H322 cells do not show significant changes in drug sensitivity. These data suggested that Tetrahydrouridine can sensitize some pancreatic and lung carcinoma cells to Gemcitabine-induced cell death regardless of CDA expression levels. Tetrahydrouridine inhibits S-phase without apoptosis[1]. |
| In Vivo | Administration of 167 mg/kg Tetrahydrouridine (THU) followed by 1.0 mg/kg DAC results in death in one male and eight females. Animals surviving to scheduled termination are generally asymptomatic with no treatment related effects observed in body weights, food consumption, clinical chemistry and urinalysis for a treatment up to 1.0 mg/kg DAC in combination with 167 mg/kg Tetrahydrouridine in animals[2]. |
| Cell Assay | Cell growth for pancreatic and lung carcinoma cell lines is carried out using the colorimetric methylene blue assay in 96-well plates at a density of 5,000 cells/well. Cells are either exposed or not exposed to Tetrahydrouridine (100 µM), counting the first 12 hrs as Day 0. Mean values are calculated from three different wells in triplicates for four days[1]. |
| Animal Admin | Mice[2] CD-1 mice (male 30-38 g and female 24-31g) from are individually housed in polycarbonate cages suspended on stainless steel racks with SaniChip certified hardwood bedding. Mice are assigned to four dose groups and a vehicle control group. Animals are gavaged with DAC or its vehicle 1 hour ± 5 minutes after administration of THU or its vehicle at a dose volume of 10 mL/kg. The DAC doses are selected based on the range finding study in which the mice tolerated six oral doses (2x/week) of 0.1, 0.2 and 0.4 mg/kg DAC in combination with a fixed dose of 167 mg/kg THU. A fixed Tetrahydrouridine dose (500 mg/m2) and the optimal timing between Tetrahydrouridine and DAC administration (60 min) are selected. Conversion of milligrams per body surface area dose in mice into milligrams per kilogram body weight dose estimation is based on Michaelis constant (km) values for mice obtained from US Food and Drug Administration published guidelines. In brief, the mouse dose in milligrams per body surface area (500 mg/m2) is divided by the km of 3 to convert the dose to milligrams per kilogram body weight (167 mg/kg). |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 668.6±55.0 °C at 760 mmHg |
| Molecular Formula | C9H16N2O6 |
| Molecular Weight | 248.233 |
| Flash Point | 358.2±31.5 °C |
| Exact Mass | 248.100830 |
| PSA | 130.33000 |
| LogP | -1.85 |
| Vapour Pressure | 0.0±4.6 mmHg at 25°C |
| Index of Refraction | 1.632 |
| Storage condition | 2-8℃ |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
|
~% 
18771-50-1 |
| Literature: US4017606 A1, ; |
|
~% 
18771-50-1 |
| Literature: US4017606 A1, ; |
| Precursor 1 | |
|---|---|
| DownStream 0 | |