501951-42-4
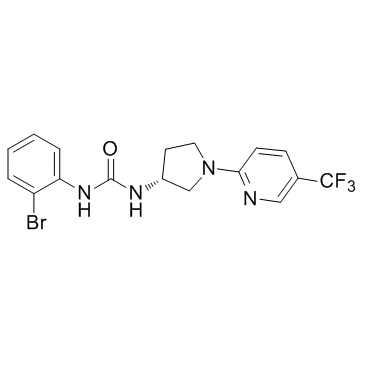
| Name | 1-(2-bromophenyl)-3-[(3R)-1-[5-(trifluoromethyl)pyridin-2-yl]pyrrolidin-3-yl]urea |
|---|---|
| Synonyms |
UNII-T74V9O0Y2W
cc-315 CS-0757 1-(2-Bromophenyl)-3-{(3R)-1-[5-(trifluoromethyl)-2-pyridinyl]-3-pyrrolidinyl}urea 1-(2-bromophenyl)-3-{(3R)-1-[5-(trifluoromethyl)pyridin-2-yl]pyrrolidin-3-yl}urea SB-705498 |
| Description | SB-705498 is a potent, selective and orally bioavailable transient receptor potential vanilloid 1 (TRPV1) receptor antagonist with a pIC50 of 7.1. |
|---|---|
| Related Catalog | |
| Target |
pIC50: 7.1 |
| In Vitro | SB705498 (0.3 nM-1 μM) potently inhibits capsaicin-induced activation of human TRPV1 expressed in 1321N1 cells or HEK293 cells with apparent pKi of 7.5 or 7.6, respectively. Coapplication of 100 nM SB705498 rapidly, completely and reversibly inhibits hTRPV1 expressed in HEK293 cells. SB705498 has no significant effect on endogenous [Ca2+] responses in HEK293 cells produced by muscarinic acetylcholine receptor activation with carbachol or store-operated channel-mediated Ca2+ entry after depletion of intracellular stores with the Ca2+ pump inhibitor thapsigargin. SB705498 (10 pM-1 μM) also has no significant antagonist effect versus the close TRPV1 receptor paralog TRPV4 transiently expressed in HEK293 cells and activated using the synthetic ligand 4α-phorbol-12,13-didecanoate (10 μM). SB705498 reveals good antagonist potency against both the rat and guinea pig TRPV1. SB705498 antagonizes rat and guinea pig TRPV1 with pKi of 7.5 and 7.3, respectively. Coapplication of 100 nM to 10 μM SB705498 to the steady state of a maintained capsaicin response leads to rapid and complete inhibition of hTRPV1 at -70 mV. SB705498 inhibits capsaicin-mediated activation of hTRPV1 with IC50 of 3 nM and 17 nM at positive and negative holding potentials (-70 mV and + 70 mV), respectively. Coapplication of 1 μM SB705498 to the plateau period of the response produces complete and reversible inhibition of the TRPV1-mediated conductance[1]. SB705498 shows approximately equal activity versus multiple and diverse chemical and physical modes of TRPV1 receptor activation. SB705498 shows little or no activity versus a wide range of ion channels, receptors and enzymes. SB705498 produces full blockade of heat as well as pH activation of hTRPV1[2]. |
| In Vivo | SB705498 exhibits potent and reversible blockade against the multiple modes of TRPV1 activation, namely the vanilloid (capsaicin), heat- and acid-mediated activation of the receptor. SB705498 displays excellent activity at 10 and 30 mg/kg po with good reversal of allodynia. SB705498 (10 mg/kg p.o.) gives 80% reversal of allodynia in the guinea pig FCA model[2]. |
| References |
| Density | 1.6±0.1 g/cm3 |
|---|---|
| Boiling Point | 506.9±50.0 °C at 760 mmHg |
| Molecular Formula | C17H16BrF3N4O |
| Molecular Weight | 429.234 |
| Flash Point | 260.4±30.1 °C |
| Exact Mass | 428.045959 |
| PSA | 60.75000 |
| LogP | 4.11 |
| Vapour Pressure | 0.0±1.3 mmHg at 25°C |
| Index of Refraction | 1.617 |
| Storage condition | -20℃ |