40918-51-2
| Name | L-Norvaline ethyl ester hydrochloride |
|---|---|
| Synonyms |
ZY3&VO2 &&L or S Form HCl
(2S)-2-Aminopentanoic acid ethyl ester hydrochloride ethyl (2S)-2-aminopentanoate,hydrochloride Ethyl L-norvalinate hydrochloride (1:1) Ethyl (S)-2-Aminopentanoate Hydrochloride (S)-2-Aminovaleric Acid Ethyl Ester Hydrochloride L-Norvaline ethyl ester hydrochloride L-Norvaline, ethyl ester, hydrochloride (1:1) MFCD08458628 Ethyl (S)-2-Aminovalerate Hydrochloride H-Nva-OEt·HCl (S)-2-Aminopentanoic acid ethyl ester hydrochloride H-NVA-OEt . HCl H-Nva-OEt.HCl |
| Description | L-Norvaline ethyl ester HCl is a valine derivative[1]. |
|---|---|
| Related Catalog | |
| In Vitro | Amino acids and amino acid derivatives have been commercially used as ergogenic supplements. They influence the secretion of anabolic hormones, supply of fuel during exercise, mental performance during stress related tasks and prevent exercise induced muscle damage. They are recognized to be beneficial as ergogenic dietary substances[1]. |
| References |
| Boiling Point | 175.4ºC at 760 mmHg |
|---|---|
| Melting Point | 104-106ºC |
| Molecular Formula | C7H16ClNO2 |
| Molecular Weight | 181.660 |
| Flash Point | 48.4ºC |
| Exact Mass | 181.086960 |
| PSA | 52.32000 |
| LogP | 2.17920 |
| Storage condition | Store at 0-5°C |
|
~% 
40918-51-2 |
| Literature: Karanfil, Abdullah; Balta, Berrin; Eskici, Mustafa Tetrahedron, 2012 , vol. 68, # 49 p. 10218 - 10229,12 Title/Abstract Full Text Show Details Karanfil, Abdullah; Balta, Berrin; Eskici, Mustafa Tetrahedron, 2012 , vol. 68, # 49 p. 10218 - 10229 |
|
~% 
40918-51-2 |
| Literature: Meppen, Malte; Pacini, Barbara; Bazzo, Renzo; Koch, Uwe; Leone, Joseph F.; Koeplinger, Kenneth A.; Rowley, Michael; Altamura, Sergio; Di Marco, Annalise; Fiore, Fabrizio; Giuliano, Claudio; Gonzalez-Paz, Odalys; Laufer, Ralph; Pucci, Vincenzo; Narjes, Frank; Gardelli, Cristina European Journal of Medicinal Chemistry, 2009 , vol. 44, # 9 p. 3765 - 3770 |
| Precursor 2 | |
|---|---|
| DownStream 1 | |



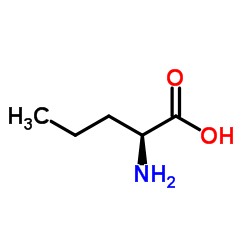
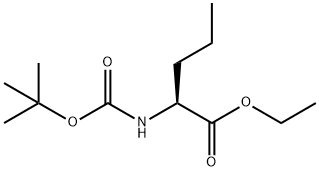
![N-[(2S)-1-Ethoxy-1-oxo-2-pentanyl]-L-alanine structure](https://image.chemsrc.com/caspic/156/82834-12-6.png)