1258-84-0
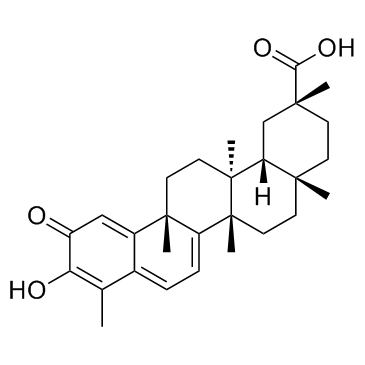
| Name | Pristimerin |
|---|---|
| Synonyms |
Methyl (2R,4aS,6aS,12bR,14aS,14bR)-10-hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydro-2-picenecarboxylate
Methyl (2R,4aS,6aS,12bR,14aS,14bR)-10-hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydropicene-2-carboxylate Methyl (2R,4aS,12bR,14aS,14bR)-10-hydroxy-2,4a,6a,9,12b,14a-hexamethyl-11-oxo-1,2,3,4,4a,5,6,6a,11,12b,13,14,14a,14b-tetradecahydro-2-picenecarboxylate Celastrol-methylether pristimerine |
| Description | Pristimerin is a potent and reversible monoacylglycerol lipase (MGL) inhibitor with an IC50 of 93 nM. |
|---|---|
| Related Catalog | |
| Target |
IC50: 93 nM (MGL)[1] |
| In Vitro | Pristimerin inhibits the activity of purified MGL with an IC50 of 93±8 nM and that of non-purified MGL (cell lysates of MGL-transfected HeLa cells) with an IC50 of 398±68 nM. Pristimerin inhibits MGL through a mechanism that is rapid, reversible and non-competitive. The binding of pristimerin to MGL might be strengthened by formation of a polar interaction with a regulatory cysteine, possibly Cys208[1]. Pristimerin inhibits HFLS-RA and HUVEC cell viability in a dose- and time-dependent manner. Pristimerin decreases VEGF-induced autophosphorylation of VEGFR2 and attenuates the activation of the VEGF-induced VEGFR2-mediated signaling pathway [2]. |
| In Vivo | Pristimerin inhibits inflammation and tumor angiogenesis. Pristimerin significantly reduces vessel density in synovial membrane tissues of inflamed joints and reduces the expression of pro-angiogenic factors in sera, including TNF-α, Ang-1, and MMP-9[2]. |
| Cell Assay | HFLS-RA (5 × 103 cells/mL) or HUVECs (1 × 104 cells/well) are seeded in 96-well plates and cultured in normal growth medium for 24 h. The cells are then incubated with different Pristimerin concentrations (0, 0.125, 0.25, 0.5 μM). The effects of Pristimerin on HUVECs viability are determined under VEGF-induced conditions. Cell viability is quantified by MTT assay. At 4 h before the end of the culture period, 30 μL of MTT solution (5.0 mg/mL) is added to each well. Cells without Pristimerin or VEGF served as a vehicle control[2]. |
| Animal Admin | Rat: Pristimerin is dissolved in DMSO (0.4%) and intraperitoneally injected daily into Male Sprague-Dawley rats in the experimental group (low-dose group, 0.40 mg/kg of body weight; high-dose group, 0.80 mg/kg of body weight) from day 11 to day 24 of immunization. The model group received vehicle (DMSO, 0.4%), and the normal control group received normal saline (NS). Methotrexate (positive control) is suspended in NS and orally administered in the autoimmune phase at an interval of 5 days[2]. |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 607.7±55.0 °C at 760 mmHg |
| Melting Point | 219.5°C |
| Molecular Formula | C30H40O4 |
| Molecular Weight | 464.636 |
| Flash Point | 195.1±25.0 °C |
| Exact Mass | 464.292664 |
| PSA | 63.60000 |
| LogP | 7.54 |
| Appearance | orange |
| Vapour Pressure | 0.0±3.9 mmHg at 25°C |
| Index of Refraction | 1.582 |
| Storage condition | Store at -20°C |
| Water Solubility | DMSO: ≥5mg/mL |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| RIDADR | NONH for all modes of transport |
|---|
|
~89% 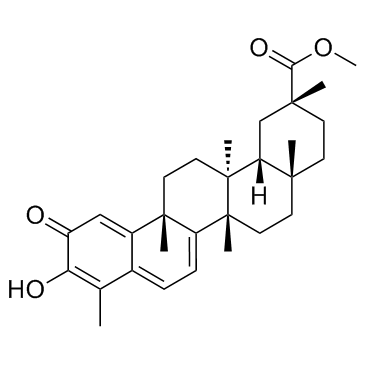
1258-84-0 |
| Literature: US2012/52019 A1, ; Page/Page column 12 ; |
|
~% 
1258-84-0 |
| Literature: Journal of the American Pharmaceutical Association (1912-1977), , vol. 28, p. 440,442 Journal of the American Pharmaceutical Association (1912-1977), , vol. 29, p. 12 Journal of the Chemical Society, , p. 4079,4087 |
| Precursor 3 | |
|---|---|
| DownStream 0 | |