405165-61-9
| Name | Besifloxacin Hydrochloride |
|---|---|
| Synonyms |
Besifloxacin HCl
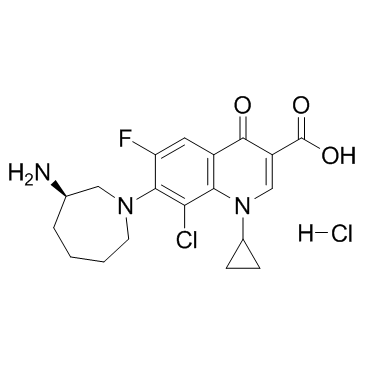
3-Quinolinecarboxylic acid, 7-[(3R)-3-aminohexahydro-1H-azepin-1-yl]-8-chloro-1-cyclopropyl-6-fluoro-1,4-dihydro-4-oxo-, hydrochloride (1:1) 7-[(3R)-3-Amino-1-azepanyl]-8-chloro-1-cyclopropyl-6-fluoro-4-oxo-1,4-dihydro-3-quinolinecarboxylic acid hydrochloride (1:1) Besifloxacin hydrochloride |
| Description | Besifloxacin hydrochloride is a fourth-generation fluoroquinolone antibiotic. IC50 Value:Target: AntibacterialBesifloxacin has been found to inhibit production of pro-inflammatory cytokines in vitro. Besifloxacin is a novel 8-chloro-fluoroquinolone agent with potent, bactericidal activity against prevalent and drug-resistant pathogens.besifloxacin is the most potent agent tested against gram-positive pathogens and anaerobes and is generally equivalent to comparator fluoroquinolones in activity against most gram-negative pathogens. Besifloxacin demonstrates potent, broad-spectrum activity, which is particularly notable against gram-positive and gram-negative isolates that are resistant to other fluoroquinolones and classes of antibacterial agents. |
|---|---|
| Related Catalog | |
| References |
| Boiling Point | 607ºC at 760 mmHg |
|---|---|
| Melting Point | >210ºC (dec.) |
| Molecular Formula | C19H22Cl2FN3O3 |
| Molecular Weight | 430.301 |
| Flash Point | 320.9ºC |
| Exact Mass | 429.102234 |
| PSA | 88.56000 |
| LogP | 4.71200 |
| Storage condition | Refrigerator |