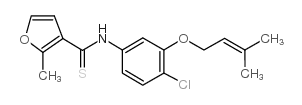
178870-32-1
| Name | N-[4-chloro-3-(3-methylbut-2-enoxy)phenyl]-2-methylfuran-3-carbothioamide |
|---|---|
| Synonyms |
2-METHYL-FURAN-3-CARBOTHIOIC ACID [4-CHLORO-3-(3-METHYL-BUT-2-ENYLOXY)-PHENYL]-AMIDE
N-[4-Chloro-3-(3-methyl-2-butenyloxy)phenyl]-2-methyl-3-furancarbothioamide Thiocarboxanilide UC-781 UC1 |
| Description | UC-781 (NSC 675186) is a highly potent and selective nonnucleoside reverse transcriptase inhibitor (NNRTI) of human immunodeficiency virus (HIV)-1 with an IC50 value of 5 nM. UC-781 is stable under low pH or various temperatures conditions. UC-781 has antiviral activity and resistance[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
HIV-1:0.005 μM (IC50) |
| In Vitro | UC-781 (0.05、0.2 和 0.5 % UC-781 补充凝胶;10 天) 从凝胶制剂中释放出来,并清除 CEM 感染细胞中的人类免疫缺陷病毒 (HIV)-1[1]。 UC-781 (3.75 -30 μM) 抑制蜡样芽孢杆菌的生长 (大约 50 %)[1]。 UC-781 抑制 CEM T 细胞中的 HIV-1 (ⅢB) (EC50=6 nM; IC50=23 nM)。UC-781 抑制单核细胞来源的树突状细胞 (MO-DC) 和自体 CD4+ T 细胞,EC50 值分别为 550 nM 和 1588 nM[2]。 UC-781 (1000 nM;24 h) 有效地防止或阻止了单核细胞来源的树突状细胞和自体 CD4+ T 细胞感染 HIV[2]。 UC-781 (0.001-1000 µM;2 h) 抑制病毒 (virus) 转移和宫颈外植体感染 HIV-1BaL[4]。 |
| In Vivo | UC-781 (100 µl 5% UC-781 补充凝胶;阴道内;一天一次持续 10 天) 从凝胶制剂中释放出来,对正常组织显示低毒性[1]。 Animal Model: Female rabbit (~1200 g; 10 weeks old)[1]. Dosage: 100 µl 5% UC-781 replens gel. Administration: Intravaginal; once daily for 10 days. Result: Released from gel preparation and had normal histology without significant increases inflammatory cells in rabbits. |
| References |
| Density | 1.24g/cm3 |
|---|---|
| Boiling Point | 440.9ºC at 760mmHg |
| Molecular Formula | C17H18ClNO2S |
| Molecular Weight | 335.84800 |
| Flash Point | 220.4ºC |
| Exact Mass | 335.07500 |
| PSA | 66.49000 |
| LogP | 5.44700 |
| Vapour Pressure | 5.68E-08mmHg at 25°C |
| Index of Refraction | 1.621 |