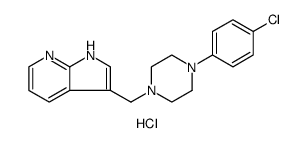
866021-03-6
| Name | 1H-Pyrrolo[2,3-b]pyridine, 3-[[4-(4-chlorophenyl)-1-piperazinyl]methyl]-, trihydrochloride |
|---|---|
| Synonyms |
L-745870 TRIHYDROCHLORIDE
L-745,870 trihydrochloride Atipamezole hydrochloride |
| Description | L-745870 trihydrochloride is a potent, selective, brain-penetrant and orally active dopamine D4 receptor antagonist with a Ki of 0.43 nM. L-745870 trihydrochloride shows weaker affinity for D2 (Ki of 960 nM) and D3 (Ki of 2300 nM) receptors, and exhibits moderate affinity for 5-HT2 receptors, sigma sites and α-adrenoceptors[1][2][3]. |
|---|---|
| Related Catalog | |
| Target |
Human D4 Receptor:4.3 nM (Ki) D2 Receptor:960 nM (Ki) D3 Receptor:2300 nM (Ki) |
| In Vitro | L-745870 is capable of antagonizing the ability of D4 receptors to inhibit agonist-induced stimulation of [35S]-GTPgS binding; blocking the inhibition of forskolin-stimulated adenylate cyclase activity in transfected human embryonic kidney (HEK293) and Chinese hamster ovary (CHO) cells; blocking dopamine-induced inhibition of Ca2+ currents in transfected GH4C1 pituitary cells; inhibiting D4 activation of cloned G protein-coupled inwardly rectifying K+ channels; and antagonizing dopamine-induced stimulation of extracellular acidification in transfected cells[1]. |
| In Vivo | L-745870 has good pharmacokinetic properties (20-60% oral bioavailability and plasma t1/2 2.1-2.8 hours) in both rat and monkey, and excellent brain penetration with high brain to plasma ratios in rat[1]. Following oral administration to squirrel monkeys, L745870 (10 mg/kg p.o.) induces mild sedation and extrapyramidal motor symptoms, notably bradykinesia, became apparent at 30 mg/kg. Lower doses of L-745870 has no observable behavioural effects in monkeys[1]. |
| References |
| Molecular Formula | C18H20Cl2N4 |
|---|---|
| Molecular Weight | 363.28400 |
| Exact Mass | 362.10700 |
| PSA | 35.16000 |
| LogP | 4.34340 |