877822-41-8
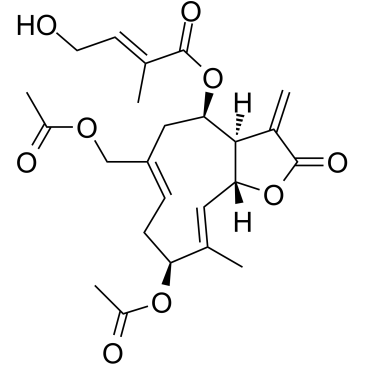
| Name | [(3aR,4R,6Z,9S,10E,11aR)-9-acetyloxy-6-(acetyloxymethyl)-10-methyl-3-methylidene-2-oxo-3a,4,5,8,9,11a-hexahydrocyclodeca[b]furan-4-yl] (E)-4-hydroxy-2-methylbut-2-enoate |
|---|---|
| Synonyms |
Eupalinolide A
(3aR,4R,6Z,9S,10E,11aR)-9-Acetoxy-6-(acetoxymethyl)-10-methyl-3-methylene-2-oxo-2,3,3a,4,5,8,9,11a-octahydrocyclodeca[b]furan-4-yl (2E)-4-hydroxy-2-methyl-2-butenoate |
| Description | Eupalinolide B is a germacrane sesquiterpene isolated from Eupatorium lindleyanum. Eupalinolide B demonstrates potent cytotoxicity against A-549, BGC-823 and HL-60 tumour cell lines[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.24±0.1 g/cm3 (20 ºC 760 Torr) |
|---|---|
| Boiling Point | 618.6±55.0 °C at 760 mmHg |
| Molecular Formula | C24H30O9 |
| Molecular Weight | 462.490 |
| Flash Point | 205.4±25.0 °C |
| Exact Mass | 462.188995 |
| PSA | 125.43000 |
| LogP | 2.50 |
| Vapour Pressure | 0.0±4.1 mmHg at 25°C |
| Index of Refraction | 1.541 |