141790-23-0
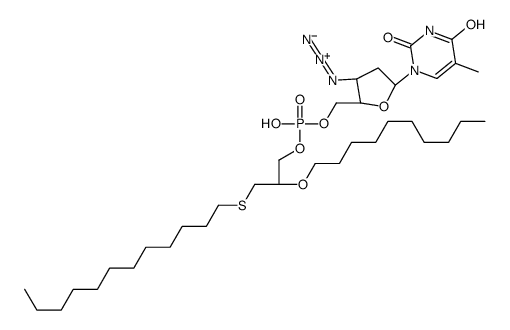
| Name | [(2S,3S,5R)-3-azido-5-(5-methyl-2,4-dioxopyrimidin-1-yl)oxolan-2-yl]methyl (2-decoxy-3-dodecylsulfanylpropyl) hydrogen phosphate |
|---|---|
| Synonyms |
fozivudine tidoxil
fosphenyloin Fosfenitoina Fosphenytoinum 3-Phosphoryloxymethyl-diphenylhydantoin HMPDP Fosphenytoine oxcarbamazepine Prodilantin Fosphenytoin 3-phosphoryloxymethyl-5,5-diphenylhydantoin |
| Description | Fozivudine tidoxil (BM-211290) is an orally active thioether lipid-zidovudine (ZDV) conjugate with anti-HIV activity. Fozivudine tidoxil, a member of the NRTI family of drug, is incorporated into the newly synthesized strand of DNA during intracellular viral replication and irreversibly binds viral RT which disrupts viral reverse-transcription[1][2]. |
|---|---|
| Related Catalog | |
| Target |
HIV |
| In Vitro | Fozivudine tidoxil (BM-211290) is a member of the nucleoside analogue reverse transcriptase inhibitor (NRTI) family[1]. |
| In Vivo | Fozivudine tidoxil (BM-211290; 45 mg/kg; PO; twice daily; one day before FIV challenge for a total of six weeks) is effective at lowering plasma- and cell-associated viremia at two weeks post-FIV infection[1]. Animal Model: Specific pathogen-free cats at 6 months of age[1] Dosage: 45 mg/kg Administration: PO; twice daily; one day before FIV challenge for a total of six weeks Result: Effective at lowering plasma- and cell-associated viremia at two weeks post-feline immunodeficiency virus (FIV) infection with a trend toward lower plasma- and cell- associated viremia at four and six weeks post-infection (PI). |
| References |
| Molecular Formula | C35H64N5O8PS |
|---|---|
| Molecular Weight | 745.95000 |
| Exact Mass | 745.42100 |
| PSA | 214.20000 |
| LogP | 8.99006 |