(2S,3R)-Voruciclib
Modify Date: 2025-08-27 14:50:55
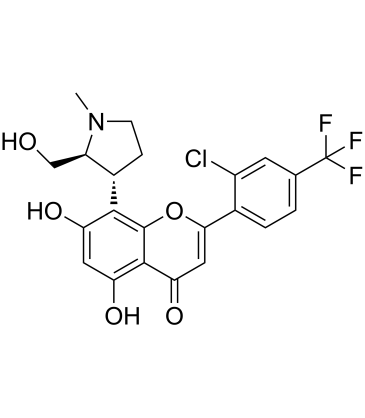
(2S,3R)-Voruciclib structure
|
Common Name | (2S,3R)-Voruciclib | ||
|---|---|---|---|---|
| CAS Number | 1253731-24-6 | Molecular Weight | 469.84 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C22H19ClF3NO5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of (2S,3R)-Voruciclib(2S,3R)-Voruciclib is the (2S,3R)-enantiomer of Voruciclib. (2S,3R)-Voruciclib is an orally active CDK inhibitor[1]. |
| Name | (2S,3R)-Voruciclib |
|---|
| Description | (2S,3R)-Voruciclib is the (2S,3R)-enantiomer of Voruciclib. (2S,3R)-Voruciclib is an orally active CDK inhibitor[1]. |
|---|---|
| Related Catalog | |
| Target |
CDK[1] |
| In Vitro | (2S,3R)-Voruciclib (Compound B, formula I) is used in combination with a compound capable of inhibiting EGFR kinase activity and Gemcitabine to treat pancreatic cancer[1]. |
| References |
| Molecular Formula | C22H19ClF3NO5 |
|---|---|
| Molecular Weight | 469.84 |
| InChIKey | MRPGRAKIAJJGMM-GXTWGEPZSA-N |
| SMILES | CN1CCC(c2c(O)cc(O)c3c(=O)cc(-c4ccc(C(F)(F)F)cc4Cl)oc23)C1CO |