| Description |
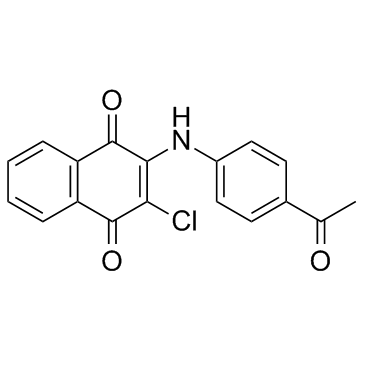
NQ301 is an antithrombotic agent; inhibits collagen-challenged rabbit platelet aggregation with an IC50 of 10 mg/mL.
|
| Related Catalog |
|
| Target |
IC50: 0.60±0.02 μM (collagen-challenged rabbit platelet aggregation), 0.58±0.04 μM (U46619-challenged rabbit platelet aggregation), 0.78±0.04 μM (arachidonic acid-challenged rabbit platelet aggregation)[1]
|
| In Vitro |
NQ301 concentration-dependently inhibits collagen (10 mg/mL)-, U46619 (1 mg/mL)- and arachidonic acid (100 mg/mL)-challenged rabbit platelet aggregation, with IC50 values of 0.60±0.02, 0.58±0.04 and 0.78±0.04 μM, respectively. NQ301 potently suppresses thromboxane B2 formation by platelets that are exposed to arachidonic acid in a concentration-dependent manner, but had no effect on the production of prostaglandin D2, indicating an inhibitory effect on thromboxane A2 synthase. NQ301 has a potential to inhibit thromboxane A2 synthase activity with thromboxane A2/prostaglandin H2 receptor blockade, and modulate arachidonic acid liberation as well as 12-hydroxy-5,8,10,14-eicosatetraenoic acid formation in platelets[1]. NQ301 inhibits platelet aggregation by suppression of the intracellular pathway, rather than by direct inhibition of fibrinogen-GPIIb/IIIa complex binding. NQ301 significantly inhibits the increase of cytosolic Ca2+ concentration and ATP secretion, and also significantly increases platelet cAMP levels in the activated platelets. The antiplatelet activity of NQ301 may be mediated by inhibition of cytosolic Ca2+ mobilization, enhancement of cAMP production and inhibition of ATP secretion in activated platelets[2].
|
| Cell Assay |
ished rabbit platelet suspension is challenged by addition of collagen (10 mg/mL), arachidonic acid (100 μM) or U46619 (1 μM). Concentration- response relationship is determined in the absence or presence of a range of concentrations of NQ301 (0, 0.25, 0.5, 0.75, 1 μM); aspirin-treated platelets (50 μM for 5 min) are used to prevent any possible contribution of endogenous arachidonic acid metabolites to platelet aggregation. The resulting aggregation, measured as the change in light transmission, is recorded for 5 min. The extent of platelet aggregation is expressed as % of the control[1].
|
| References |
[1]. Jin YR, et al. An antithrombotic agent, NQ301, inhibits thromboxane A2 receptor and synthase activity in rabbit platelets. Basic Clin Pharmacol Toxicol. 2005 Sep;97(3):162-7. [2]. Zhang YH, et al. Antiplatelet effect of 2-chloro-3-(4-acetophenyl)-amino-1,4-naphthoquinone (NQ301): a possible mechanism through inhibition of intracellular Ca2+ mobilization. Biol Pharm Bull. 2001 Jun;24(6):618-22.
|