TDN345
Modify Date: 2025-08-26 00:43:17
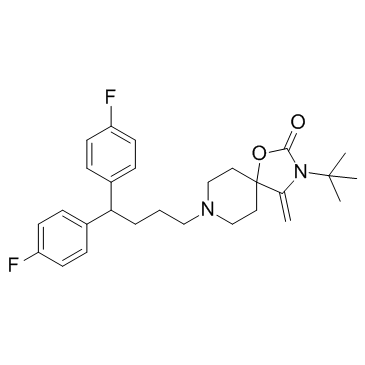
TDN345 structure
|
Common Name | TDN345 | ||
|---|---|---|---|---|
| CAS Number | 134069-68-4 | Molecular Weight | 468.57900 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C28H34F2N2O2 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of TDN345TDN345 is a Ca2+ antagonist, used for the treatment of vascular and senile dementia including Alzheimer's disease. |
| Name | 8-[4,4-bis(4-fluorophenyl)butyl]-3-tert-butyl-4-methylidene-1-oxa-3,8-diazaspiro[4.5]decan-2-one |
|---|---|
| Synonym | More Synonyms |
| Description | TDN345 is a Ca2+ antagonist, used for the treatment of vascular and senile dementia including Alzheimer's disease. |
|---|---|
| Related Catalog | |
| In Vitro | TDN-345 (10 μM) significantly increases the intracellular NGF content in the time-course study. TDN-345 induces NGF synthesis/secretion at the concentrations of 0.1 μM; statistically significant at 1 μM. The ED50 is 0.88 μM[1]. |
| In Vivo | TDN-345 (0.1-1.0 mg/kg, p.o.) dose-dependently decreases the mortality and ischemic neurological deficit score when administered orally twice, 60 min before ischemia and 90 min after recirculation. Additionally, TDN-345 (0.2 or 1.0 mg/kg, p.o. once daily for 3 weeks after the onset of stroke) decreases the mortality and recurrence of stroke in SHRSP[2]. |
| Animal Admin | Male Mongolian gerbils (50-70 g body weight) are anesthetized lightly by ether inhalation. A 1-2 cm midline throat incision provided access to both carotid arteries, which are clamped with microaneurysm clamps immediately after recovery from anesthesia. Sixty minutes before occlusion, TDN-345 (0.3 or 1.0 mg/kg suspended in a 5% gum arabic solution or 0.1 or 0.3 mg/kg with 1% NaHCO3 suspended in a 5% gum arabic solution) or vehicle is administered orally. After 15 min of bilateral carotid artery occlusion, the clamps are removed. Ninety minutes after reperfusion, TDN-345 or vehicle is again administered orally. The body temperature is maintained at 37°C during the experimental period using a heating pad. The experiments are performed in nine to 15 animals in each group. Animal survival is observed 8 h and 7 days after reperfusion, and neurological signs are evaluated according to the scoring system as an ischemic neurological score for 5 h after the ischemic insult from an area under the time-neurological deficit score curve (AUCreperfusion (0-300 min)) (hair roughed up or tremor, obtunded, paucity of move, 1; ptosis, seizure, 2; head cocked, eyes fixed open, splayed out hind limbs, extreme rotation, circling behavior, rolling seizure, 3; coma, 6; death, 34). Nine to 15 animals are used in each experimental group. |
| References |
| Molecular Formula | C28H34F2N2O2 |
|---|---|
| Molecular Weight | 468.57900 |
| Exact Mass | 468.25900 |
| PSA | 32.78000 |
| LogP | 6.35170 |
| Vapour Pressure | 2.08E-12mmHg at 25°C |
| InChIKey | OFRYIDMZALXALS-UHFFFAOYSA-N |
| SMILES | C=C1N(C(C)(C)C)C(=O)OC12CCN(CCCC(c1ccc(F)cc1)c1ccc(F)cc1)CC2 |
| Storage condition | 2-8℃ |
| 1-Oxa-3,8-diazaspiro(4.5)decan-2-one,8-(4,4-bis(4-fluorophenyl)butyl)-3-(1,1-dimethylethyl)-4-methylene |
| 8-[4,4-bis(4-fluorophenyl)butyl]-2-tert-butyl-1-methylidene-4-oxa-2,8-diazaspiro[4.5]decan-3-one |
| TDN345 |