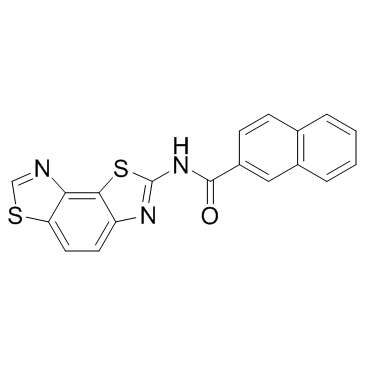
| Description |
KIN1148, a small-molecule IRF3 agonist, is a novel influenza vaccine adjuvant found to enhance flu vaccine efficacy.
|
| Related Catalog |
|
| In Vitro |
KIN1148 induces the dose-dependent nuclear translocation of IRF3 in PH5CH8 cells and specific activation of IRF3- responsive promoters. KIN1148 also elicits greater induction of endogenous IRF3-dependent ISG54 and OASL expression by PH5CH8 cells[1].
|
| In Vivo |
Prime-boost immunization of mice with a suboptimal dose of a monovalent pandemic influenza split virus H1N1 A/California/07/2009 vaccine plus KIN1148 protect against a lethal challenge with mouse-adapted influenza virus (A/California/04/2009) and induce an influenza virusspecific IL-10 and Th2 response by T cells derived from lung and lung-draining lymph nodes. Primeboost immunization with vaccine plus KIN1148, but not prime immunization alone, induce antibodies capable of inhibiting influenza virus hemagglutinin and neutralizing viral infectivity. Nevertheless, a single immunization with vaccine plus KIN1148 provide increased protection over vaccine alone and reduce viral load in the lungs after challenge[1].
|
| Animal Admin |
Mice: The final liposomes prior to mixing with vaccine contained KIN1148 (5 mg/mL) and phospholipids (40 mg/mL) and are delivered intramuscularly (KIN1148 at 50 μg and phospholipid at 400 μg per dose). Blank liposomes are prepared without KIN1148 as vehicle control. KIN1148 liposome and blank liposome formulations are stable for at least 4 months at 4°C and are used for in vivo experiments within a month after preparation[1].
|
| References |
[1]. Probst P, et al. A small-molecule IRF3 agonist functions as an influenza vaccine adjuvant by modulating the antiviral immune response. Vaccine. 2017 Apr 4;35(15):1964-1971.
|