L-732,138
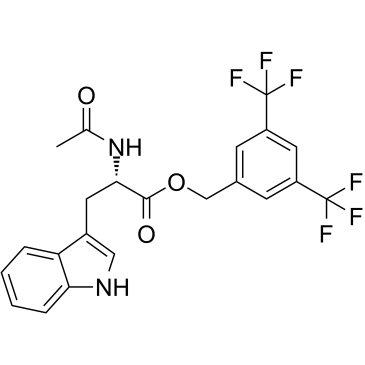
L-732,138 structure
|
Common Name | L-732,138 | ||
|---|---|---|---|---|
| CAS Number | 148451-96-1 | Molecular Weight | 472.38000 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C22H18F6N2O3 | Melting Point | 147-148 ℃(lit.) | |
| MSDS | USA | Flash Point | N/A | |
Use of L-732,138L-732138 is a selective, potent and competitive neurokinin-1 (NK-1) receptor antagonist with an IC50 of 2.3 nM. L-732138 has 200-fold more potent in cloned human NK-1 receptors than cloned rat NK-1 receptors, and has > 1000-fold more potent than human NK-2 and NK-3 receptors. L-732138 can reduce hyperalgesia and has antitumor action[1][2]. |
| Name | [3,5-bis(trifluoromethyl)phenyl]methyl (2S)-2-acetamido-3-(1H-indol-3-yl)propanoate |
|---|---|
| Synonym | More Synonyms |
| Description | L-732138 is a selective, potent and competitive neurokinin-1 (NK-1) receptor antagonist with an IC50 of 2.3 nM. L-732138 has 200-fold more potent in cloned human NK-1 receptors than cloned rat NK-1 receptors, and has > 1000-fold more potent than human NK-2 and NK-3 receptors. L-732138 can reduce hyperalgesia and has antitumor action[1][2]. |
|---|---|
| Related Catalog | |
| Target |
NK1:2.3 nM (IC50) |
| In Vitro | L-732138 (0 -100 µM; first doubling time; COLO 858, MEL HO and COLO 679 cells) treatment results in a concentration-dependent cytotoxicity. L-732138 inhibits cell growth with IC50 of 44.6 μM for COLO 858 cells, 76.3 μM for MEL HO cells and 64.2 μM for COLO 679 cells. L-732138 blocks substance P (SP) mitogen stimulation[1]. L-732,138 treatment results in a large number of apoptotic cells were found in COLO 858, MEL HO and COLO 679 melanoma cell lines. In DAPI-stained cultures, at IC50 concentration of 43.6% apoptotic cells for the three melanoma cell lines, whereas at IC100 concentration of 51.4 % apoptotic cells[1]. Cell Proliferation Assay[1] Cell Line: COLO 858, MEL HO and COLO 679 cells Concentration: 0 µM, 20 µM, 40 µM, 60 µM, 80 µM, 100 µM Incubation Time: First doubling time Result: Resulted in a concentration-dependent cytotoxicity. |
| In Vivo | L-732138 (10-4-10-2 mol/kg; intravenous injection; for 15 minutes; male Dunkin-Hartley guinea-pigs) treatment abolishes vagally-induced plasma exudation and significantly inhibits the enhancement by LPS. The LPS-enhanced vagally-induced plasma exudation is not completely inhibited by either L-732138 or SOD pretreatment alone, but is blocked by the combination of both pretreatments[3]. Animal Model: Male Dunkin-Hartley guinea-pigs (350-500 g) injected with lipopolysaccharide (LPS)[3] Dosage: 10-4 mol/kg , 10-3 mol/kg and 10-2 mol/kg Administration: Intravenous injection; for 15 minutes Result: Abolished the vagally-induced plasma leakage in tracheobronchial tissues, and dose-dependently inhibited the LPS enhanced vagally-induced plasma exudation in traceobronchial tissues. |
| References |
| Melting Point | 147-148 ℃(lit.) |
|---|---|
| Molecular Formula | C22H18F6N2O3 |
| Molecular Weight | 472.38000 |
| Exact Mass | 472.12200 |
| PSA | 71.19000 |
| LogP | 5.38700 |
| InChIKey | BYYQYXVAWXAYQC-IBGZPJMESA-N |
| SMILES | CC(=O)NC(Cc1c[nH]c2ccccc12)C(=O)OCc1cc(C(F)(F)F)cc(C(F)(F)F)c1 |
| Storage condition | 2-8°C |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
|
Role of tachykinins and neurokinin receptor subtypes in the regulation of motility of the forestomach and abomasum in conscious sheep.
Neuropeptides 47(1) , 9-18, (2013) The present study was planned to evaluate role of tachykinins (TKs) and neurokinin (NK) receptors in the regulation of gastric motility in sheep. We examined the effects of intravenous (i.v.) injectio... |
|
|
Substance P induces adverse myocardial remodelling via a mechanism involving cardiac mast cells.
Cardiovasc. Res. 92(3) , 420-9, (2011) Substance P and neurokinin A (NKA) are sensory nerve neuropeptides encoded by the TAC1 gene. Substance P is a mast cell secretagogue and mast cells are known to play a role in adverse myocardial remod... |
|
|
Paradoxical effect of salbutamol in a model of acute organophosphates intoxication in guinea pigs: role of substance P release.
Am. J. Physiol. Lung Cell. Mol. Physiol. 292(4) , L915-23, (2007) Organophosphates induce bronchoobstruction in guinea pigs, and salbutamol only transiently reverses this effect, suggesting that it triggers additional obstructive mechanisms. To further explore this ... |
| L-732,138 |
| Carbamoylcholine chloride |
| N-Acetyl-L-trp-3,5-bistrifluoromethylbenzyl ester |
| L-732138 |