Tolvaptan
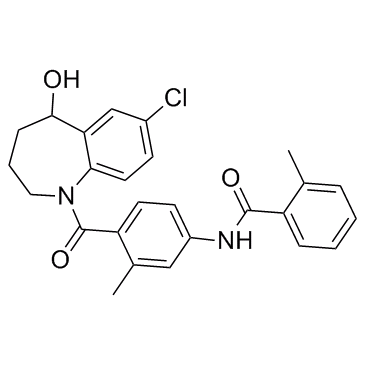
Tolvaptan structure
|
Common Name | Tolvaptan | ||
|---|---|---|---|---|
| CAS Number | 150683-30-0 | Molecular Weight | 448.941 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 594.4±50.0 °C at 760 mmHg | |
| Molecular Formula | C26H25ClN2O3 | Melting Point | 219-222°C | |
| MSDS | Chinese USA | Flash Point | 313.3±30.1 °C | |
Use of TolvaptanTolvaptan is a selective, competitive arginine vasopressin receptor 2 antagonist with an IC50 of 1.28μM for the inhibition of AVP-induced platelet aggregation.IC50 value: 1.28 uM (inhibition of AVP-induced platelet aggregation)Target: vasopressin receptor 2Tolvaptan (OPC-41061) is a selective, competitive arginine vasopressin receptor 2 antagonist with an IC50 of 1.28μM for the inhibition of AVP-induced platelet aggregation. Tolvaptan (OPC-41061) is used to treat hyponatremia (low blood sodium levels) associated with congestive heart failure, cirrhosis, and the syndrome of inappropriate antidiuretic hormone (SIADH). Tolvaptan (OPC-41061) is also in fast-track clinical trials for polycystic kidney disease. Treatment with t tolvaptan (OPC-41061) causes rapid and sustained body weight reductions concurrent with increases in urine output, improves and/or normalizes serum sodium in hyponatremic patients, reduces signs and symptoms of congestion and increases thirst. However, tolvaptan (OPC-41061) has not been shown to decrease heart failure re-hospitalization or mortality. As an adjunct to standard therapy, tolvaptan (OPC-41061) is unique in that it is virtually the only novel agent tested in patients hospitalized for acute heart failure syndrome (AHFS) to reach its primary end point for short-term efficacy without causing deleterious side effects. |
| Name | N-[4-(7-chloro-5-hydroxy-2,3,4,5-tetrahydro-1-benzazepine-1-carbonyl)-3-methylphenyl]-2-methylbenzamide |
|---|---|
| Synonym | More Synonyms |
| Description | Tolvaptan is a selective, competitive arginine vasopressin receptor 2 antagonist with an IC50 of 1.28μM for the inhibition of AVP-induced platelet aggregation.IC50 value: 1.28 uM (inhibition of AVP-induced platelet aggregation)Target: vasopressin receptor 2Tolvaptan (OPC-41061) is a selective, competitive arginine vasopressin receptor 2 antagonist with an IC50 of 1.28μM for the inhibition of AVP-induced platelet aggregation. Tolvaptan (OPC-41061) is used to treat hyponatremia (low blood sodium levels) associated with congestive heart failure, cirrhosis, and the syndrome of inappropriate antidiuretic hormone (SIADH). Tolvaptan (OPC-41061) is also in fast-track clinical trials for polycystic kidney disease. Treatment with t tolvaptan (OPC-41061) causes rapid and sustained body weight reductions concurrent with increases in urine output, improves and/or normalizes serum sodium in hyponatremic patients, reduces signs and symptoms of congestion and increases thirst. However, tolvaptan (OPC-41061) has not been shown to decrease heart failure re-hospitalization or mortality. As an adjunct to standard therapy, tolvaptan (OPC-41061) is unique in that it is virtually the only novel agent tested in patients hospitalized for acute heart failure syndrome (AHFS) to reach its primary end point for short-term efficacy without causing deleterious side effects. |
|---|---|
| Related Catalog | |
| References |
[2]. Inomata T.Tolvaptan (vasopressin receptor antagonist).Nihon Rinsho. 2011 Nov;69 Suppl 9:408-11. |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 594.4±50.0 °C at 760 mmHg |
| Melting Point | 219-222°C |
| Molecular Formula | C26H25ClN2O3 |
| Molecular Weight | 448.941 |
| Flash Point | 313.3±30.1 °C |
| Exact Mass | 448.155365 |
| PSA | 69.64000 |
| LogP | 4.09 |
| Appearance of Characters | white to tan |
| Vapour Pressure | 0.0±1.8 mmHg at 25°C |
| Index of Refraction | 1.664 |
| Storage condition | Refrigerator |
| Water Solubility | DMSO: ≥15mg/mL |
| RIDADR | NONH for all modes of transport |
|---|
| Precursor 9 | |
|---|---|
| DownStream 1 | |
|
Relation of serum uric acid levels and outcomes among patients hospitalized for worsening heart failure with reduced ejection fraction (from the efficacy of vasopressin antagonism in heart failure outcome study with tolvaptan trial).
Am. J. Cardiol. 114(11) , 1713-21, (2014) We investigated the clinical profiles associated with serum uric acid (sUA) levels in a large cohort of patients hospitalized for worsening chronic heart failure with ejection fraction (EF) ≤40%, with... |
|
|
Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model.
J. Am. Soc. Nephrol. 26(1) , 39-47, (2015) Autosomal dominant polycystic kidney disease (ADPKD) is a leading cause of ESRD. A central defect associated with ADPKD pathology is elevated levels of 3', 5'-cyclic AMP (cAMP). Compounds such as tolv... |
|
|
Association of arginine vasopressin levels with outcomes and the effect of V2 blockade in patients hospitalized for heart failure with reduced ejection fraction: insights from the EVEREST trial.
Circ. Heart Fail. 6(1) , 47-52, (2013) Arginine vasopressin (AVP) levels are elevated in proportion to heart failure severity and are associated with higher cardiovascular mortality in ambulatory patients. However, the relationship between... |
| (±)-4'-[(7-chloro-2,3,4,5-tetrahydro-5-hydroxy-1H-1-benzazepin-1-yl)carbonyl]-o-tolu-m-toluidide |
| Tolvaptan |
| UNII-21G72T1950 |
| olvaptan |
| N-{4-[(7-Chloro-5-hydroxy-2,3,4,5-tetrahydro-1H-1-benzazepin-1-yl)carbonyl]-3-methylphenyl}-2-methylbenzamide |
| Benzamide, N-[4-[(7-chloro-2,3,4,5-tetrahydro-5-hydroxy-1H-1-benzazepin-1-yl)carbonyl]-3-methylphenyl]-2-methyl- |
| Samsca |
| benzazepine derivative,32 |
| N-[4-(9-chloro-6-hydroxy-2-azabicyclo[5.4.0]undeca-8,10,12-triene-2-carbonyl)-3-methyl-phenyl]-2-methyl-benzamide |
![N-[4-[(7-Chloro-2,3,4,5-tetrahydro-5-oxo-1H-1-benzazepin-1-yl)carbonyl]-3-methylphenyl]-2-methylbenzamide Structure](https://image.chemsrc.com/caspic/486/137973-76-3.png) CAS#:137973-76-3
CAS#:137973-76-3 CAS#:30459-70-2
CAS#:30459-70-2 CAS#:160129-45-3
CAS#:160129-45-3 CAS#:137982-91-3
CAS#:137982-91-3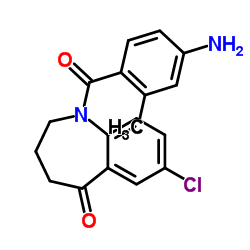 CAS#:137977-97-0
CAS#:137977-97-0![2,2-dimethylpropionic acid 7-chloro-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl ester Structure](https://image.chemsrc.com/caspic/182/863762-10-1.png) CAS#:863762-10-1
CAS#:863762-10-1![2,2-dimethylpropionic acid 4-[(E)-hydroxyimino]-7-chloro-1,2,3,4-tetrahydronaphthalen-1-yl ester Structure](https://image.chemsrc.com/caspic/313/863762-02-1.png) CAS#:863762-02-1
CAS#:863762-02-1![2,2-dimethylpropionic acid 7-chloro-1-(4-amino-2-methylbenzoyl)-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl ester Structure](https://image.chemsrc.com/caspic/323/863762-24-7.png) CAS#:863762-24-7
CAS#:863762-24-7![2,2-dimethylpropionic acid 7-chloro-1-(2-methyl-4-nitrobenzoyl)-2,3,4,5-tetrahydro-1H-benzo[b]azepin-5-yl ester Structure](https://image.chemsrc.com/caspic/475/863762-20-3.png) CAS#:863762-20-3
CAS#:863762-20-3
