2-Phenylacetic dithioperoxyanhydride
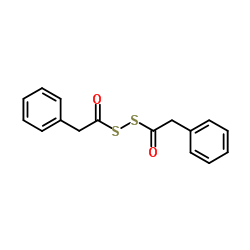
2-Phenylacetic dithioperoxyanhydride structure
|
Common Name | 2-Phenylacetic dithioperoxyanhydride | ||
|---|---|---|---|---|
| CAS Number | 15088-78-5 | Molecular Weight | 302.411 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 481.1±48.0 °C at 760 mmHg | |
| Molecular Formula | C16H14O2S2 | Melting Point | 59-63 °C(lit.) | |
| MSDS | Chinese USA | Flash Point | 210.1±19.6 °C | |
| Name | Phenylacetyl disulfide |
|---|---|
| Synonym | More Synonyms |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 481.1±48.0 °C at 760 mmHg |
| Melting Point | 59-63 °C(lit.) |
| Molecular Formula | C16H14O2S2 |
| Molecular Weight | 302.411 |
| Flash Point | 210.1±19.6 °C |
| Exact Mass | 302.043518 |
| PSA | 84.74000 |
| LogP | 4.68 |
| Vapour Pressure | 0.0±1.2 mmHg at 25°C |
| Index of Refraction | 1.638 |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| Safety Phrases | S22-S24/25 |
| RIDADR | NONH for all modes of transport |
| WGK Germany | 2 |
| HS Code | 2930909090 |
|
~95% 
2-Phenylacetic ... CAS#:15088-78-5 |
| Literature: Kodomari, Mitsuo; Fukuda, Masaharu; Yoshitomi, Suehiko Synthesis, 1981 , # 8 p. 637 - 638 |
|
~% 
2-Phenylacetic ... CAS#:15088-78-5 |
| Literature: Journal of Organic Chemistry, , vol. 42, # 13 p. 2224 - 2229 |
|
~% 
2-Phenylacetic ... CAS#:15088-78-5 |
| Literature: Journal of the American Chemical Society, , vol. 28, p. 1459 |
| HS Code | 2930909090 |
|---|---|
| Summary | 2930909090. other organo-sulphur compounds. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
|
Synthesis and biological activity of PTEN-resistant analogues of phosphatidylinositol 3,4,5-trisphosphate.
J. Am. Chem. Soc. 128(51) , 16464-5, (2006) The activation of phosphatidylinositol 3-kinase (PI 3-K) and subsequent production of PtdIns(3,4,5)P3 launches a signal transduction cascade that impinges on a plethora of downstream effects on cell p... |
|
|
Synthesis of antisense oligonucleotides: Replacement of 3H-1, 2-benzodithiol-3-one 1, 1-dioxide (Beaucage reagent) with phenylacetyl disulfide (PADS) as efficient sulfurization reagent: From bench to bulk manufacture of active pharmaceutical ingredient. Cheruvallath ZS, et al.
Org. Process Res. Dev. 4(3) , 199-204, (2000)
|
|
|
Use of phenylacetyl disulfide (PADS) in the synthesis of oligodeoxyribonucleotide phosphorothioates. Cheruvallath ZS, et al.
Nucleosides Nucleotides Nucleic Acids 18(3) , 485-492, (1999)
|
| Bis(phenylacetyl) Disulfide |
| PADS |
| bis-phenylacetyl-disulfane |
| 2-Phenylacetic dithioperoxyanhydride |
| DIPHENYLACETYL DISULFIDE |
| Diphenacetyldisulfid |
| Phenylacetyl Disulfide |
| PHENYLACETYL DISULPHIDE |
| Bis-phenylacetyl-disulfan |
| MFCD03453048 |
| Dibenzyldithioperoxyanhydride |
| Bis-phenylacetyl-disulfid |
| Phenylacetyldisulfide |
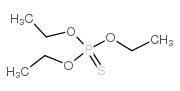 CAS#:126-68-1
CAS#:126-68-1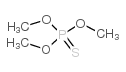 CAS#:152-18-1
CAS#:152-18-1
