Helioxanthin derivative 5-4-2
Modify Date: 2025-09-20 21:56:53
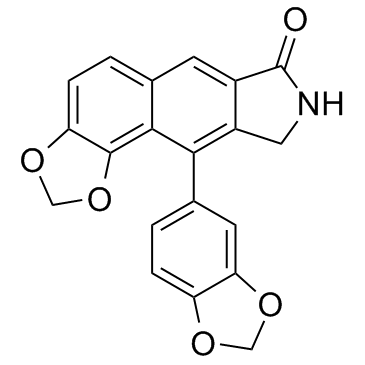
Helioxanthin derivative 5-4-2 structure
|
Common Name | Helioxanthin derivative 5-4-2 | ||
|---|---|---|---|---|
| CAS Number | 203935-39-1 | Molecular Weight | 347.32100 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C20H13NO5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of Helioxanthin derivative 5-4-2Helioxanthin derivative 5-4-2 is an analogue of helioxanthin, exhibites significant in vitro anti-HBV activity with EC50 of 0.08 uM in HepG2.2.15 cells.IC50 value: 0.08 uM (EC50) [1][2]Target: Anti-HBVHelioxanthin derivative 5-4-2 had potent anti-HBV activities in HepG2.2.15 cells, with the EC50s of 1 and 0.08 microM, respectively. The lamivudine-resistant HBV, L526M/M550V double mutant strain, was also sensitive to helioxanthin and 5-4-2. This class of compounds not only inhibited HBV DNA, but also decreased HBV mRNA and HBV protein expression. The EC50 of HBV DNA inhibition was consistent with the EC50 of HBV 3.5 Kb transcript inhibition, which was 1 and 0.09 microM for helioxanthin and 5-4-2 respectively. |
| Name | 10-(1,3-benzodioxol-5-yl)-8,9-dihydro-7H-[1,3]benzodioxolo[4,5-f]isoindol-7-one |
|---|---|
| Synonym | More Synonyms |
| Description | Helioxanthin derivative 5-4-2 is an analogue of helioxanthin, exhibites significant in vitro anti-HBV activity with EC50 of 0.08 uM in HepG2.2.15 cells.IC50 value: 0.08 uM (EC50) [1][2]Target: Anti-HBVHelioxanthin derivative 5-4-2 had potent anti-HBV activities in HepG2.2.15 cells, with the EC50s of 1 and 0.08 microM, respectively. The lamivudine-resistant HBV, L526M/M550V double mutant strain, was also sensitive to helioxanthin and 5-4-2. This class of compounds not only inhibited HBV DNA, but also decreased HBV mRNA and HBV protein expression. The EC50 of HBV DNA inhibition was consistent with the EC50 of HBV 3.5 Kb transcript inhibition, which was 1 and 0.09 microM for helioxanthin and 5-4-2 respectively. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C20H13NO5 |
|---|---|
| Molecular Weight | 347.32100 |
| Exact Mass | 347.07900 |
| PSA | 66.02000 |
| LogP | 3.53640 |
| InChIKey | UVKXTQRJKHBAMT-UHFFFAOYSA-N |
| SMILES | O=C1NCc2c1cc1ccc3c(c1c2-c1ccc2c(c1)OCO2)OCO3 |
| Storage condition | 2-8℃ |
| 10-benzo[1,3]dioxol-5-yl-8,9-dihydro-1,3-dioxa-8-aza-dicyclopenta[a,g]naphthalen-7-one |
| 10-(1,3-benzodioxol-5-yl)-8,9-dihydro-7H-1,3-benzodioxolo[4,5-f]isoindol-7-one |
| Helioxanthin derivative 5-4-2 |