Purvalanol A
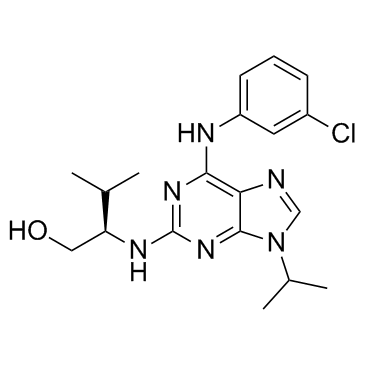
Purvalanol A structure
|
Common Name | Purvalanol A | ||
|---|---|---|---|---|
| CAS Number | 212844-53-6 | Molecular Weight | 388.894 | |
| Density | 1.3±0.1 g/cm3 | Boiling Point | 590.5±60.0 °C at 760 mmHg | |
| Molecular Formula | C19H25ClN6O | Melting Point | N/A | |
| MSDS | Chinese USA | Flash Point | 310.9±32.9 °C | |
Use of Purvalanol APurvalanol A is a potent CDK inhibitor, which inhibits cdc2-cyclin B, cdk2-cyclin A, cdk2-cyclin E, cdk4-cyclin D1, and cdk5-p35 with IC50s of 4, 70, 35, 850, 75 nM, resepctively. |
| Name | purvalanol A |
|---|---|
| Synonym | More Synonyms |
| Description | Purvalanol A is a potent CDK inhibitor, which inhibits cdc2-cyclin B, cdk2-cyclin A, cdk2-cyclin E, cdk4-cyclin D1, and cdk5-p35 with IC50s of 4, 70, 35, 850, 75 nM, resepctively. |
|---|---|
| Related Catalog | |
| Target |
cdc2-cyclin B:4 nM (IC50) cdk2-cyclin E:35 nM (IC50) cdk2-cyclin A:70 nM (IC50) cdk4-cyclin D1:850 nM (IC50) cdk5-p35:75 nM (IC50) erk1:9000 nM (IC50) |
| In Vitro | Purvalanol A inhibits cdc28 (S. cerevisiae) and erk1 with IC50s of 80 and 9000 nM. Purvalanol A shows inhibitory activities against the NCI panel of 60 human tumor cell lines, with average GI50 of 2 μM; two cell lines show an ∼20-fold increase in sensitivity to purvalanol A: the KM12 colon cancer cell line with a GI50 of 76 nM and the NCI-H522 non–small cell lung cancer cell line with a GI50 of 347 nM[1]. Purvalanol A is a 2.5-fold more potent inhibitor of CDK2, but also inhibits DYRK1A potently and a number of other protein kinases in the low micromolar range. Purvalanol A inhibits MKK1, MAPK2/ERK2, JNK/SAPK1c with IC50s of 80, 26, 84 μM[2]. Purvalanol A selectively inhibits the phosphorylation of cellular proteins. Purvalanol A prevents the increases of the contents of cyclins D and E during serum-induced G1 phase progression. Purvalanol A does not inhibit transcription under cell-free conditions[3]. |
| References |
| Density | 1.3±0.1 g/cm3 |
|---|---|
| Boiling Point | 590.5±60.0 °C at 760 mmHg |
| Molecular Formula | C19H25ClN6O |
| Molecular Weight | 388.894 |
| Flash Point | 310.9±32.9 °C |
| Exact Mass | 388.177826 |
| PSA | 87.89000 |
| LogP | 2.77 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.654 |
| Storage condition | −20°C |
| Water Solubility | methylene chloride: 50 mg/mL, clear, colorless |
| Personal Protective Equipment | Eyeshields;Gloves;type N95 (US);type P1 (EN143) respirator filter |
|---|---|
| RIDADR | NONH for all modes of transport |
| WGK Germany | 3 |
| HS Code | 2933990090 |
| HS Code | 2933990090 |
|---|---|
| Summary | 2933990090. heterocyclic compounds with nitrogen hetero-atom(s) only. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:20.0% |
|
Phosphoproteomic Analysis of Aurora Kinase Inhibition in Monopolar Cytokinesis.
J. Proteome Res. 14 , 4087-98, (2015) Cytokinesis is the last step of the cell cycle that requires coordinated activities of the microtubule cytoskeleton, actin cytoskeleton, and membrane compartments. Aurora B kinase is one of the master... |
|
|
Involvement of atypical transcription factor E2F8 in the polyploidization during mouse and human decidualization.
Cell Cycle 14 , 1842-58, (2015) Polyploid decidual cells are specifically differentiated cells during mouse uterine decidualization. However, little is known about the regulatory mechanism and physiological significance of polyploid... |
|
|
KAP regulates ROCK2 and Cdk2 in an RNA-activated glioblastoma invasion pathway.
Oncogene 34(11) , 1432-41, (2015) Aberrant splicing of the cyclin-dependent kinase-associated phosphatase, KAP, promotes glioblastoma invasion in a Cdc2-dependent manner. However, the mechanism by which this occurs is unknown. Here we... |
| P01 |
| 2-(1R-Isopropyl-2-hydroxyethylamino)-6-(3-chloroanilino)-9-isopropylpurine NG-60 |
| (2R)-2-[[6-(3-chloroanilino)-9-propan-2-ylpurin-2-yl]amino]-3-methylbutan-1-ol |
| NG-60 |
| (2R)-2-({6-[(3-Chlorophenyl)amino]-9-isopropyl-9H-purin-2-yl}amino)-3-methyl-1-butanol |
| (2R)-2-({6-[(3-Chlorophenyl)amino]-9-isopropyl-9H-purin-2-yl}amino)-3-methylbutan-1-ol |
| 1-Butanol, 2-[[6-[(3-chlorophenyl)amino]-9-(1-methylethyl)-9H-purin-2-yl]amino]-3-methyl-, (2R)- |
| Purvalanol A |
| Purv |