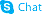H-Glu[cyclo(-Arg-Gly-Asp-D-Phe-Lys)]-cyclo(-Arg-Gly-Asp-D-Phe-Lys) trifluoroacetate salt
Modify Date: 2023-01-26 20:15:50
![H-Glu[cyclo(-Arg-Gly-Asp-D-Phe-Lys)]-cyclo(-Arg-Gly-Asp-D-Phe-Lys) trifluoroacetate salt Structure](https://www.chemsrc.com/caspic/207/250612-47-6.png)
H-Glu[cyclo(-Arg-Gly-Asp-D-Phe-Lys)]-cyclo(-Arg-Gly-Asp-D-Phe-Lys) trifluoroacetate salt structure
|
Common Name | H-Glu[cyclo(-Arg-Gly-Asp-D-Phe-Lys)]-cyclo(-Arg-Gly-Asp-D-Phe-Lys) trifluoroacetate salt | ||
|---|---|---|---|---|
| CAS Number | 250612-47-6 | Molecular Weight | 1318.46 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C59H87N19O16 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of H-Glu[cyclo(-Arg-Gly-Asp-D-Phe-Lys)]-cyclo(-Arg-Gly-Asp-D-Phe-Lys) trifluoroacetate saltE(c(RGDfK))??is an αvβ3 integrin-specific binding moiety with tumor targeting properties. Increased uptake of E(c(RGDfK))??in human ovarian cancer OVCAR-3 xenograft tumors may be useful in cancer research[1]. |
| Name | H-Glu[cyclo(-Arg-Gly-Asp-D-Phe-Lys)]-cyclo(-Arg-Gly-Asp-D-Phe-Lys) trifluoroacetate salt |
|---|
| Description | E(c(RGDfK))??is an αvβ3 integrin-specific binding moiety with tumor targeting properties. Increased uptake of E(c(RGDfK))??in human ovarian cancer OVCAR-3 xenograft tumors may be useful in cancer research[1]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C59H87N19O16 |
|---|---|
| Molecular Weight | 1318.46 |