7-O-Methyl-6-Prenylnaringenin
Modify Date: 2025-08-21 13:23:34
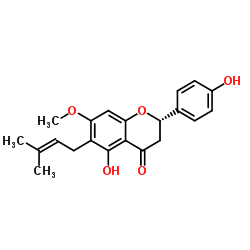
7-O-Methyl-6-Prenylnaringenin structure
|
Common Name | 7-O-Methyl-6-Prenylnaringenin | ||
|---|---|---|---|---|
| CAS Number | 261776-61-8 | Molecular Weight | 354.396 | |
| Density | 1.2±0.1 g/cm3 | Boiling Point | 585.8±50.0 °C at 760 mmHg | |
| Molecular Formula | C21H22O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 208.6±23.6 °C | |
Use of 7-O-Methyl-6-Prenylnaringenin7-O-Methyl-6-Prenylnaringenin is an active compound. 7-O-Methyl-6-Prenylnaringenin can be isolated from Humulus lupulus[1]. |
| Name | (2S)-5-Hydroxy-2-(4-hydroxyphenyl)-7-methoxy-6-(3-methyl-2-buten-1-yl)-2,3-dihydro-4H-chromen-4-one |
|---|---|
| Synonym | More Synonyms |
| Description | 7-O-Methyl-6-Prenylnaringenin is an active compound. 7-O-Methyl-6-Prenylnaringenin can be isolated from Humulus lupulus[1]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.2±0.1 g/cm3 |
|---|---|
| Boiling Point | 585.8±50.0 °C at 760 mmHg |
| Molecular Formula | C21H22O5 |
| Molecular Weight | 354.396 |
| Flash Point | 208.6±23.6 °C |
| Exact Mass | 354.146729 |
| LogP | 5.47 |
| Vapour Pressure | 0.0±1.7 mmHg at 25°C |
| Index of Refraction | 1.608 |
| InChIKey | PMQBKFKEJGCJIK-KRWDZBQOSA-N |
| SMILES | COc1cc2c(c(O)c1CC=C(C)C)C(=O)CC(c1ccc(O)cc1)O2 |
| 4H-1-Benzopyran-4-one, 2,3-dihydro-5-hydroxy-2-(4-hydroxyphenyl)-7-methoxy-6-(3-methyl-2-buten-1-yl)-, (2S)- |
| (2S)-5-Hydroxy-2-(4-hydroxyphenyl)-7-methoxy-6-(3-methyl-2-buten-1-yl)-2,3-dihydro-4H-chromen-4-one |