BMS-1166-N-piperidine-CO-N-piperazine dihydrochloride
Modify Date: 2025-08-28 19:42:24
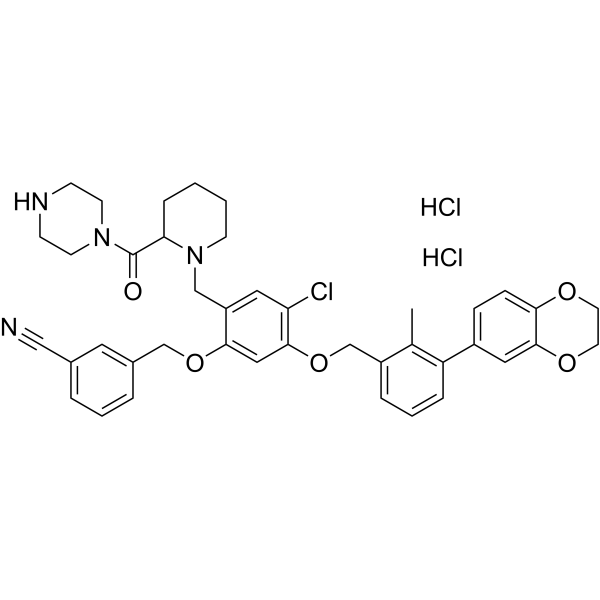
BMS-1166-N-piperidine-CO-N-piperazine dihydrochloride structure
|
Common Name | BMS-1166-N-piperidine-CO-N-piperazine dihydrochloride | ||
|---|---|---|---|---|
| CAS Number | 2691796-83-3 | Molecular Weight | 780.18 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C41H45Cl3N4O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of BMS-1166-N-piperidine-CO-N-piperazine dihydrochlorideBMS-1166-N-piperidine-CO-N-piperazine dihydrochloride incorporates a ligand for PD-1/PD-L1 immune checkpoint, and a PROTAC linker. BMS-1166-N-piperidine-CO-N-piperazine dihydrochloride can be used in the synthesis of PD-1/PD-L1 degrader-1.html" class="link-product" target="_blank">PROTAC PD-1/PD-L1 degrader-1 (HY-131183). PROTAC PD-1/PD-L1 degrader-1 inhibits PD-1/PD-L1 interaction with an IC50 of 39.2 nM[1]. |
| Name | BMS-1166-N-piperidine-CO-N-piperazine dihydrochloride |
|---|
| Description | BMS-1166-N-piperidine-CO-N-piperazine dihydrochloride incorporates a ligand for PD-1/PD-L1 immune checkpoint, and a PROTAC linker. BMS-1166-N-piperidine-CO-N-piperazine dihydrochloride can be used in the synthesis of PD-1/PD-L1 degrader-1.html" class="link-product" target="_blank">PROTAC PD-1/PD-L1 degrader-1 (HY-131183). PROTAC PD-1/PD-L1 degrader-1 inhibits PD-1/PD-L1 interaction with an IC50 of 39.2 nM[1]. |
|---|---|
| Related Catalog | |
| In Vitro | PROTAC 包含两个不同的配体,通过一个接头连接;一个是 E3 泛素连接酶的配体,另一个是靶蛋白的配体。PROTAC 利用细胞内泛素-蛋白酶体系统选择性降解靶蛋白[1]。 |
| References |
| Molecular Formula | C41H45Cl3N4O5 |
|---|---|
| Molecular Weight | 780.18 |
| InChIKey | GHDAHXKKICFPOZ-UHFFFAOYSA-N |
| SMILES | Cc1c(COc2cc(OCc3cccc(C#N)c3)c(CN3CCCCC3C(=O)N3CCNCC3)cc2Cl)cccc1-c1ccc2c(c1)OCCO2.Cl.Cl |