Acetic acid,2-(4-chloro-2-methylphenoxy)-, ethyl ester
Modify Date: 2024-01-14 21:34:01
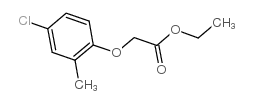
Acetic acid,2-(4-chloro-2-methylphenoxy)-, ethyl ester structure
|
Common Name | Acetic acid,2-(4-chloro-2-methylphenoxy)-, ethyl ester | ||
|---|---|---|---|---|
| CAS Number | 2698-38-6 | Molecular Weight | 228.67200 | |
| Density | 1.174g/cm3 | Boiling Point | 300.4ºC at 760mmHg | |
| Molecular Formula | C11H13ClO3 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 118.7ºC | |
| Name | MCPA-ethyl |
|---|---|
| Synonym | More Synonyms |
| Density | 1.174g/cm3 |
|---|---|
| Boiling Point | 300.4ºC at 760mmHg |
| Molecular Formula | C11H13ClO3 |
| Molecular Weight | 228.67200 |
| Flash Point | 118.7ºC |
| Exact Mass | 228.05500 |
| PSA | 35.53000 |
| LogP | 2.59030 |
| Vapour Pressure | 0.00112mmHg at 25°C |
| Index of Refraction | 1.511 |
| Storage condition | 0-6°C |
CHEMICAL IDENTIFICATION
HEALTH HAZARD DATAACUTE TOXICITY DATA
|
| HS Code | 2918990090 |
|---|
|
~% 
Acetic acid,2-(... CAS#:2698-38-6 |
| Literature: Newman; Fones; Renoll Journal of the American Chemical Society, 1947 , vol. 69, p. 718,721 |
|
~% 
Acetic acid,2-(... CAS#:2698-38-6 |
| Literature: Sandham, David A.; Aldcroft, Clive; Baettig, Urs; Barker, Lucy; Beer, David; Bhalay, Gurdip; Brown, Zarin; Dubois, Gerald; Budd, David; Bidlake, Louise; Campbell, Emma; Cox, Brian; Everatt, Brian; Harrison, David; Leblanc, Catherine J.; Manini, Jodie; Profit, Rachael; Stringer, Rowan; Thompson, Katy S.; Turner, Katharine L.; Tweed, Morris F.; Walker, Christoph; Watson, Simon J.; Whitebread, Steven; Willis, Jennifer; Williams, Gareth; Wilson, Caroline Bioorganic and Medicinal Chemistry Letters, 2007 , vol. 17, # 15 p. 4347 - 4350 |
| HS Code | 2918990090 |
|---|---|
| Summary | 2918990090. other carboxylic acids with additional oxygen function and their anhydrides, halides, peroxides and peroxyacids; their halogenated, sulphonated, nitrated or nitrosated derivatives. VAT:17.0%. Tax rebate rate:13.0%. . MFN tariff:6.5%. General tariff:30.0% |
| ethyl (4-chloro-2-methylphenoxy)acetate |
| Ethyl 2-methyl-4-chlorophenoxyacetate |
| Mcpee |
| ethyl 2-(4-chloro-2-methylphenoxy)acetate |
| ethyl 4-chloro-o-tolyloxyacetate |
| Ethyl 4-chloro-o-tolyloxyacetate |
| MCPA ethyl ester |

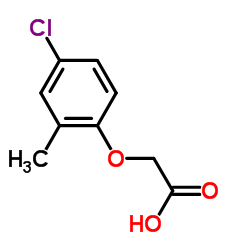
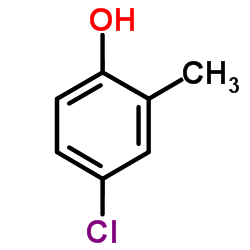
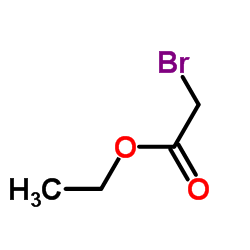
 CAS#:7399-57-7
CAS#:7399-57-7