8-iso Prostaglandin E2
Modify Date: 2024-01-11 18:55:06
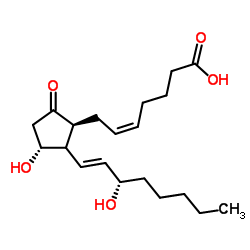
8-iso Prostaglandin E2 structure
|
Common Name | 8-iso Prostaglandin E2 | ||
|---|---|---|---|---|
| CAS Number | 27415-25-4 | Molecular Weight | 352.465 | |
| Density | 1.1±0.1 g/cm3 | Boiling Point | 530.1±50.0 °C at 760 mmHg | |
| Molecular Formula | C20H32O5 | Melting Point | N/A | |
| MSDS | N/A | Flash Point | 288.5±26.6 °C | |
Use of 8-iso Prostaglandin E28-Isoprostaglandin E2 (iPE2-III) is a member of the isoprostane class of prostanoids. 8-Isoprostaglandin E2 acts at the receptor for thromboxane A2 (the TP) in vivo to induce vasoconstriction and platelet aggregation. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway[1][2]. |
| Name | 8-iso Prostaglandin E2 |
|---|---|
| Synonym | More Synonyms |
| Description | 8-Isoprostaglandin E2 (iPE2-III) is a member of the isoprostane class of prostanoids. 8-Isoprostaglandin E2 acts at the receptor for thromboxane A2 (the TP) in vivo to induce vasoconstriction and platelet aggregation. 8-Isoprostaglandin E2 enhances receptor-activated NFkappa B ligand (RANKL)-dependent osteoclastic potential of marrow hematopoietic precursors via the cAMP pathway[1][2]. |
|---|---|
| Related Catalog | |
| References |
| Density | 1.1±0.1 g/cm3 |
|---|---|
| Boiling Point | 530.1±50.0 °C at 760 mmHg |
| Molecular Formula | C20H32O5 |
| Molecular Weight | 352.465 |
| Flash Point | 288.5±26.6 °C |
| Exact Mass | 352.224976 |
| PSA | 94.83000 |
| LogP | 1.88 |
| Vapour Pressure | 0.0±3.2 mmHg at 25°C |
| Index of Refraction | 1.561 |
| l-7-(3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl)-5-heptenoic acid |
| (5E,13E)-11,15-Dihydroxy-9-oxoprosta-5,13-dien-1-oic acid |
| 5-Heptenoic acid, 7-[3-hydroxy-2- (3-hydroxy-1-octenyl)-5-oxocyclopentyl]- |
| (5Z,8β,11α,12ξ,13E,15S)-11,15-Dihydroxy-9-oxoprosta-5,13-dien-1-oic acid |
| 5-Heptenoic acid, 7-(3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl)- |
| 7-(3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl)-5-heptenoic acid |
| (5E)-7-{3-Hydroxy-2-[(1E)-3-hydroxy-1-octen-1-yl]-5-oxocyclopentyl}-5-heptenoic acid |
| Prosta-5,13-dien-1-oic acid, 11,15-dihydroxy-9-oxo-, (5Z,8β,11α,12ξ,13E,15S)- |
| 5-Heptenoic acid, 7-(3-hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl)-, l- |
| Prosta-5,13-dien-1-oic acid, 11,15-dihydroxy-9-oxo-, (5E,13E)- |
| 7-[3-Hydroxy-2-(3-hydroxy-1-octenyl)-5-oxocyclopentyl]-5-heptenoic acid |