Tanaproget
Modify Date: 2025-08-26 19:04:04
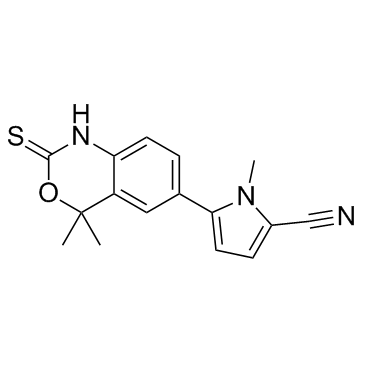
Tanaproget structure
|
Common Name | Tanaproget | ||
|---|---|---|---|---|
| CAS Number | 304853-42-7 | Molecular Weight | 297.37500 | |
| Density | N/A | Boiling Point | N/A | |
| Molecular Formula | C16H15N3OS | Melting Point | N/A | |
| MSDS | N/A | Flash Point | N/A | |
Use of TanaprogetTanaproget(NSP989) is a novel nonsteroidal progesterone receptor agonist which can bind to the PR from various species with a higher relative affinity than reference steroidal progestins.IC50 value: 0.1 nM (EC50, induce alkaline phosphatase activity) [1]Target: progesterone receptorTanaproget represents a potential first-in-class nonsteroidal PR agonist for contraception with improved safety and side effect profiles versus currently available steroidal oral contraceptives.in vitro: In T47D cells, TNPR induces alkaline phosphatase activity with an EC(50) value of 0.1 nm, comparable with potent steroidal progestins such as medroxyprogesterone acetate (MPA) and trimegestone (TMG), albeit with a reduced efficacy ( approximately 60%). In a mammalian two-hybrid assay to measure PR agonist-induced interaction between steroid receptor co-activator-1 and PR, TNPR showed similar potency (EC(50) value of 0.02 nm) and efficacy to MPA and TMG [1].in vivo: TNPR effectively down-regulated MMP expression in vitro and induced significant reduction of lesions in mice with disease established by tissues from endometriosis patients [2]. The maximum concentration (C(max)) of tanaproget occurred approximately 2 to 3 h after administration. The elimination half-life (t(1/2)) ranged from 12 to 30 h, and the oral clearance was approximately 70 L/h. The pharmacokinetics of tanaproget was not noticeably altered with a high-fat meal [3].Toxicity: All doses of tanaproget decreased cervical mucus scores (using a modified Insler method), indicating poor production and poor quality of cervical mucus. The most frequent treatment-emergent adverse events were vaginal bleeding/spotting, abdominal cramping and vomiting; their incidence was not dose related and most events were mild [3]. |
| Name | 5-(4,4-dimethyl-2-sulfanylidene-1H-3,1-benzoxazin-6-yl)-1-methylpyrrole-2-carbonitrile |
|---|---|
| Synonym | More Synonyms |
| Description | Tanaproget(NSP989) is a novel nonsteroidal progesterone receptor agonist which can bind to the PR from various species with a higher relative affinity than reference steroidal progestins.IC50 value: 0.1 nM (EC50, induce alkaline phosphatase activity) [1]Target: progesterone receptorTanaproget represents a potential first-in-class nonsteroidal PR agonist for contraception with improved safety and side effect profiles versus currently available steroidal oral contraceptives.in vitro: In T47D cells, TNPR induces alkaline phosphatase activity with an EC(50) value of 0.1 nm, comparable with potent steroidal progestins such as medroxyprogesterone acetate (MPA) and trimegestone (TMG), albeit with a reduced efficacy ( approximately 60%). In a mammalian two-hybrid assay to measure PR agonist-induced interaction between steroid receptor co-activator-1 and PR, TNPR showed similar potency (EC(50) value of 0.02 nm) and efficacy to MPA and TMG [1].in vivo: TNPR effectively down-regulated MMP expression in vitro and induced significant reduction of lesions in mice with disease established by tissues from endometriosis patients [2]. The maximum concentration (C(max)) of tanaproget occurred approximately 2 to 3 h after administration. The elimination half-life (t(1/2)) ranged from 12 to 30 h, and the oral clearance was approximately 70 L/h. The pharmacokinetics of tanaproget was not noticeably altered with a high-fat meal [3].Toxicity: All doses of tanaproget decreased cervical mucus scores (using a modified Insler method), indicating poor production and poor quality of cervical mucus. The most frequent treatment-emergent adverse events were vaginal bleeding/spotting, abdominal cramping and vomiting; their incidence was not dose related and most events were mild [3]. |
|---|---|
| Related Catalog | |
| References |
| Molecular Formula | C16H15N3OS |
|---|---|
| Molecular Weight | 297.37500 |
| Exact Mass | 297.09400 |
| PSA | 89.11000 |
| LogP | 3.18198 |
| InChIKey | PYVFWTPEBMRKSR-UHFFFAOYSA-N |
| SMILES | Cn1c(C#N)ccc1-c1ccc2c(c1)C(C)(C)OC(=S)N2 |
| Storage condition | 2-8℃ |
|
~73% 
Tanaproget CAS#:304853-42-7 |
| Literature: Wyeth Patent: US2005/228179 A1, 2005 ; Location in patent: Page/Page column 7-8 ; |
|
~% 
Tanaproget CAS#:304853-42-7 |
| Literature: Fensome, Andrew; Bender, Reinhold; Chopra, Rajiv; Cohen, Jeff; Collins, Mark A.; Hudak, Valerie; Malakian, Karl; Lockhead, Susan; Olland, Andrea; Svenson, Kristine; Terefenko, Eugene A.; Unwalla, Ray J.; Wilhelm, James M.; Wolfrom, Scott; Zhu, Yuan; Zhang, Zhiming; Zhang, Puwen; Winneker, Richard C.; Wrobel, Jay Journal of Medicinal Chemistry, 2005 , vol. 48, # 16 p. 5092 - 5095 |
| Precursor 1 | |
|---|---|
| DownStream 0 | |
| Tanaproget |
| UNII-W9F9H8GXWR |
| 1zuc |
| NSP-989 |

![5-(4,4-dimethyl-2-oxo-2,4-dihydro-1H-benzo[d][1,3]oxazin-6-yl)-1H-pyrrole-2-carbonitrile structure](https://image.chemsrc.com/caspic/441/304853-99-4.png)